Isolation and characterization of GqDNVs
Briefly, GqDNVs were purified from fresh goji berries (Lycium barbarum L., obtained from Zhongwei, Ningxia, via cold-chain transportation) via a sucrose density gradient centrifugation method following our laboratory’s previous publication [23]. GqDNVs were then suspended in phosphate-buffered saline (PBS) and filtered through a 0.22 μm filter to remove impurities (Fig. 1a). Before use, the size and concentration of GqDNVs were examined by nanoparticle tracking analysis (NTA) via a NanoSight NS300 (Malvern Instruments Ltd., UK). The morphology of the GqDNVs was visualized via transmission electron microscopy (TEM, HT-7700, Hitachi, Japan). The contents of GqDNVs were subsequently detected via untargeted and saccharides-targeted metabolomic sequencing via the UPLC–MS/MS and the GC–MS platform, respectively.
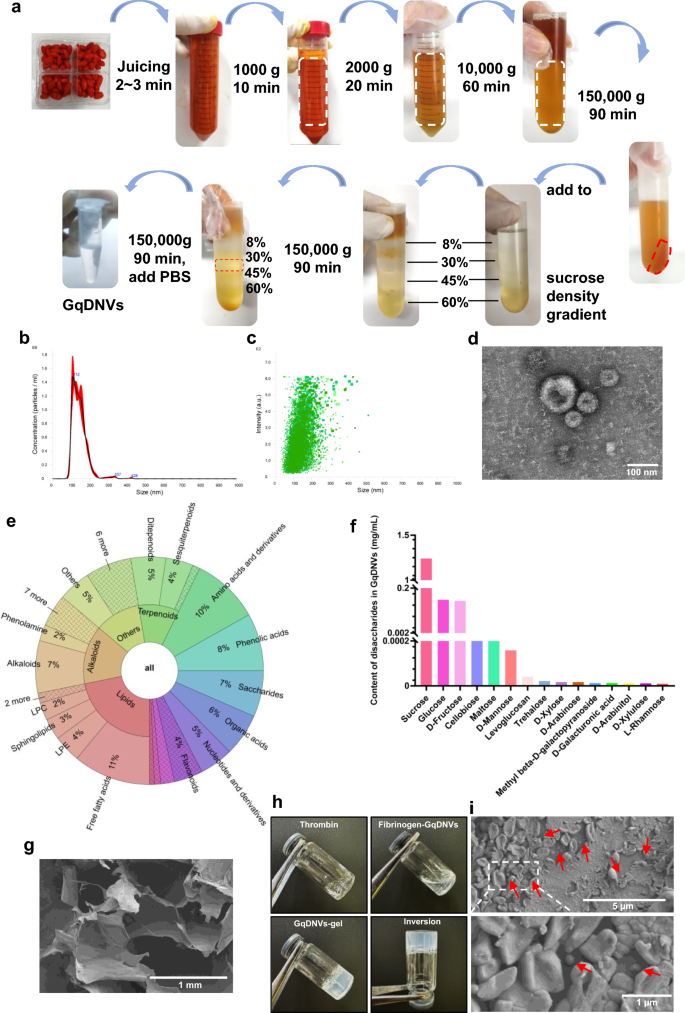
Preparation and characterization of GqDNVs and GqDNVs-gel. a GqDNVs were isolated and purified using sucrose density gradient ultracentrifugation. b The distribution of particle size and concentration of GqDNVs was analyzed. c A scatter plot showing the intensity distribution of GqDNVs. d Representative transmission electron microscopy image showing the morphology of GqDNVs. e GqDNVs content was analysed by non-targeted metabolome sequencing. f The saccharides content of GqDNVs was assessed through saccharides-targeted metabolome sequencing. g Scanning electron micrograph of the fibrin gel. h GqDNVs-gel preparation process. i Scanning electron microscopy reveals GqDNVs encapsulated within the fibrin gel. White arrows indicate encapsulated GqDNVs. GqDNVs: Gouqi-derived nanovesicles, GqDNVs-gel: Gouqi-derived nanovesicles-fibrin gel
Generation of GqDNVs-gel
GqDNVs-fibrin gel was prepared according to previously published methods for exosome-loaded fibrin [29, 30]. A defined amount of GqDNVs was added to the fibrinogen solution and mixed with thrombin to form GqDNVs-gel. GqDNVs were integrated into the fibrinogen solution by mixing a 10 mg/mL fibrinogen solution (dissolved in PBS) with a 1 × 1010 particles/mL GqDNVs solution (dissolved in PBS). Then, the GqDNVs-rich fibrinogen solution and thrombin solution were mixed to quickly form a stable gel patch. The morphologies of the fibrin gel and GqDNVs-gel were characterized via the Scanning electron microscope (SEM, HITACHI S4800).
Cell culture
HL-1 cells (SCC065) were obtained from EMD Millipore, Shanghai, China. The cells were cultured in minimum essential medium (MEM) supplemented with 10% fetal bovine serum (Pricella Life Science & Technology Co., Ltd, China) at 37 °C in a 5% CO2 humidified atmosphere. Hypoxia was induced using the AnaeroPack System (Mitsubishi Gas Chemical Co., Ltd) and glucose-free Earle’s buffer (Gibco). Dehydrocorydaline chloride (DHC) (300 nM, MedChemExpress, China) [31] and SB 203580 (SB) (10 μM, MedChemExpress, China) [32] were used to activate and inhibit the activity of p38 MAPK, respectively.
Cardiomyocyte biocompatibility with GqDNVs-gel
To evaluate the impact of each component of the GqDNVs-gel on cardiomyocytes, HL-1 cells were seeded in 96-well plates and cultured for 24 h. Subsequently, the medium was replaced with fresh medium containing 5 µL of either fibrin gel, GqDNVs solution, or GqDNVs-gel, ensuring that the GqDNVs concentration was consistent across the GqDNVs solution and GqDNVs-gel. Following a 12-h co-incubated period, the viability of the HL-1 cells was assessed using the Cell Counting Kit-8 (CCK-8, Beyotime, China) assay to determine the cytotoxicity associated with each component of the GqDNVs-gel.
GqDNVs uptake assay
To evaluate the uptake of GqDNVs by cardiomyocytes, we initially labelled GqDNVs with PKH26 (MedChemExpress, China) and subsequently prepared a GqDNVs-gel. HL-1 cells were co-incubated with PBS, PKH26-prelabelled GqDNVs, or PKH26-prelabelled GqDNVs-gel for 6 h. The cytoskeleton and nucleus of the cells were stained with Phalloidin (MaoKang, China) and 4’,6-diamidino-2-phenylindole (DAPI) (Beyotime, China), respectively. The samples were subsequently analysed using confocal fluorescence microscopy (FV3000, OMTOOLS, China).
In vivo and ex vivo imaging of GqDNVs distribution in mouse hearts
To assess the feasibility of using GqDNVs-gel for the delivery of GqDNVs, we administered an equivalent dose of DiR (AAT Bioquest, USA)-prelabelled GqDNVs via a myocardial GqDNVs drop, GqDNVs injection, or GqDNVs-gel to the hearts of C57BL/6 mice. At 2-, 12-, and 24-h post-administration, the distribution of GqDNVs in live mice and their hearts was evaluated using the Lago X optical imaging systems (SI Imaging, USA). The fluorescence intensities within the region of interest (ROI) were quantified using Aura imaging software (SI Imaging, USA).
Mouse model of MI
Eight-week-old male C57BL/6 mice were procured from Vital River Laboratories in Beijing, China. Following international standards, all animal care and experiments were approved by the Institutional Animal Care and Use Committee, Huazhong University of Science and Technology (IACUC Number: 3626). After one week of adaptive feeding, an acute MI model was established in the mice following protocols detailed in prior studies [33, 34]. In summary, ischemia was induced through permanent ligation of the left anterior descending coronary artery. Post-MI, the mice received either PBS or GqDNVs-gel (containing 1 × 108 particles of GqDNVs), with 35 mice in each group.
Cardiac echocardiography
A blinded veterinary cardiologist performed transthoracic echocardiography at 4 h and 14 days post-MI using the VisualSonics Vevo 1100 system (FUJIFILM, China). Mice from each experimental group were randomly selected and anesthetized using a 1–3% isoflurane mixture in 95% oxygen. Parasternal left ventricular long-axis images were subsequently recorded. M-mode echocardiography was employed to assess various cardiac parameters, including left ventricular (LV) mass, left ventricular ejection fraction (LVEF), left ventricular short-axis fractional shortening (LVFS), left ventricular end-diastolic volume (LVEdV), left ventricular end-systolic volume (LVEsV), heart rate, QRS duration, and cardiac output.
Heart histology
Following euthanasia, the hearts were excised and processed for paraffin embedding according to established procedures. For hematoxylin–eosin (HE) staining, hearts were sliced into 4 μm-thick sections and stained with the HE staining kit (G1005, Servicebio). For Masson’s staining, hearts from the apex to the ligation level were sectioned at a thickness of 5 μm and stained with a Masson’s staining kit (G1006, Servicebio). Microscopic images were captured, and morphometric parameters, including infarct size and infarct wall thickness, were quantified using ImageJ software.
Immunofluorescence
Immunofluorescence was performed on paraffin-embedded heart cryosections and HL-1 cells. Heart cryosections were blocked in 3% BSA at room temperature for 30 min, then incubated with a mixture of primary antibodies against rabbit anti-p38 (phospho T180 + Y182) (1:100 dilution, ab195049, Abcam) and mouse anti-alpha sarcomeric actin (α-SMA) (1:200, 67,735-1-Ig, Proteintech), mouse anti-CD31 (1:200 dilution, GB12063, Servicebio), rabbit anti-proliferation marker protein Ki-67 (Ki67) (1:100 dilution, GB111499, Servicebio), and mouse anti-proliferating cell nuclear antigen (PCNA) (1:100 dilution, GB12010, Servicebio) at 4 °C overnight. After washing, the sections were incubated with appropriate secondary antibodies (goat anti-rabbit IgG (Alexa Fluor 488, GB25303, Servicebio) or goat anti-Mouse IgG (Alexa Fluor 594, 115–585-003, Jackson) at room temperature for 60 min, followed by counterstaining with DAPI (G1012, Servicebio) for 10 min in the darkroom.
HL-1 cells inoculated in 12-well plates after the indicated treatments were fixed with 4% paraformaldehyde for 10 min. After washing, HL-1 cells were co-incubated with 0.1% Triton and 5% BSA for 2 h at room temperature, followed by incubation with a mixture of primary antibodies against rabbit anti-p38 (phospho T180 + Y182) (1:100 dilution, ab195049, Abcam), rabbit anti-Phospho-NF-κB p65 (1:100 dilution, AP1294, ABclonal), rabbit anti- Ki-67 (1:100 dilution, GB111499, Servicebio), and mouse anti- PCNA (1:100 dilution, GB12010, Servicebio) at 4 °C overnight. Goat anti-rabbit IgG (Alexa Fluor 594, ab150080, Abcam) was used as the secondary antibody for one hour of incubation at room temperature. Subsequently, F-actin was stained with FITC-labelled phalloidin (1:1000 dilution, MX4404, Maokangbio) for 30 min at room temperature. After washing, the sections were incubated with appropriate secondary antibodies (goat anti-rabbit IgG (Alexa Fluor 488, GB25303, Servicebio) or goat anti-mouse IgG (Alexa Fluor 594, 115-585-003, Jackson)) at room temperature for 60 min, followed by counterstaining with DAPI (G1012, Servicebio) for 10 min in the darkroom.
For terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) staining, heart cryosections or HL-1 cells were incubated using the TMR (red) TUNEL Cell Apoptosis Detection Kit (G1502, Servicebio). Following washing, DAPI (G1012, Servicebio) was used for nuclear staining. All washes (3 × 3 min) between steps were performed in PBS. All images were taken with a confocal fluorescence microscope (FV3000, OMTOOLS, China).
Quantitative real-time polymerase chain reaction (q-PCR)
Total RNA extraction was carried out using the RNA Easy Fast (DP451, TIANGEN) following the manufacturer’s instructions. Reverse transcription was performed using the FastKing gDNA Dispelling RT SuperMix (KR118, TIANGEN). The q-PCR was performed with the FastKing OneStep RT-PCR Kit (KR123, TIANGEN). Relative gene expression was obtained and analysed with a Quant Studio 7 Flex Real-Time PCR system (Applied Biosystems Thermo Fisher Scientific).
Western blotting
Total protein was extracted from the infarcted region of heart tissue. A 10% SDS–polyacrylamide gel was used to separate the target proteins under a constant voltage of 150 V for electrophoresis. Subsequently, the target proteins were transferred onto a nitrocellulose (NC) membrane, which was then blocked with a universal blocking solution for 15 min. The NC membrane was incubated with the appropriate primary antibodies overnight on a shaker at 4 °C: p38 (phospho T180 + Y182) (ab170099, Abcam), p38 alpha/MAPK14 (ab195049, Abcam), Phospho-ERK1/2 (Thr202/Tyr204) (AF1015, Affinity Biosciences), ERK1/2 (AF0155, Affinity Biosciences), Phospho-JNK1/2/3 (Thr183 + Tyr185) (AF3318, Affinity Biosciences), JNK1/2/3 (Thr183 + Tyr185) (AF6318, Affinity Biosciences), Phospho-NF-κB p65 (AP1294, ABclonal), NF-κB p65 (A19653, ABclonal), Cleaved Caspase-7 (8438S, Cell signaling), Caspase-7 (12827S, Cell signaling), Cleaved Caspase-3 (9664S, Cell signaling), Caspase-3 (9662S, Cell signaling), Bax (ET1603-34, Huabio), Bcl-2 (3498S, Cell signaling), and GAPDH (GB15004, Servicebio). On the second day, the NC membrane was treated with corresponding secondary antibodies for one hour at room temperature: Anti-rabbit IgG, HRP-linked Antibody (7074, Cell Signaling), and Anti-mouse IgG, HRP-linked Antibody (7076, Cell signaling). The targeted protein expression levels were calculated by ImageJ and normalized to GAPDH.
Enzyme-linked immunosorbent assay (ELISA)
Serum samples were collected after centrifuging the coagulated blood at 3000g for 15 min. The levels of transforming growth factor beta 2 (TGFβ-2, E-EL-M1191, Elabscience Biotechnology Co., Ltd, China), insulin-like growth factor 1 (IGF-1, E-EL-M3006, Elabscience Biotechnology Co., Ltd, China), and vascular endothelial growth factor A (VEGF-A, E-MSEL-M0005, Elabscience Biotechnology Co., Ltd, China) in the serum were measured according to the manufacturer’s instructions.
Transcriptome RNA sequencing and analysis
A total amount of l μg RNA per sample of heart tissue was used as input material for the RNA sample preparations. The sequencing libraries were prepared using the NEBNextRUltraTMRNA Library Prep Kit for Illumina (NEB, USA). After cluster generation by the TruSeq PE Cluster Kit v3-cBot-HS (Illumina), the library preparations were sequenced on an Illumina platform. Gene alignment was calculated using featureCounts. The enrichment analysis is performed based on the hypergeometric test. A heat map of differentially expressed genes was generated using the online tool Morpheus. Genes whose fold change (FC) ≥ 2 or ≤ 0.5, variable importance in projection (VIP) > 1, and P-value ≤ 0.05 were considered significantly different. Differential expression analysis between the two groups was performed by the DESeq2 package. Gene set enrichment analysis (GSEA) was performed using GSEA v3.0 software (Broad Institute). All genes included in the DESeq2 output were mapped to HUGO Gene Nomenclature Committee symbols and ranked according to the following: − log10 (padj) for upregulated genes and (− 1) × − log10 (padj) for downregulated genes. Gene Ontology biological processes were obtained from the Molecular Signatures Database. GSEA was run in classic prerank mode with 1000 permutations to assess the false discovery rate (FDR). A weighted enrichment score was used, and the gene set size was limited to 15 to 500 genes. Gene sets with an FDR < 0.05 were defined as significantly enriched.
Proteomic analysis
The 4D-data-independent acquisition (DIA) quantitative proteomics analysis utilized the diaPASEF acquisition mode of the timsTOF Pro2 series mass spectrometer (MS) to achieve differential quantitative proteomic analysis. The samples were subjected to a series of processes, including protein extraction, enzyme digestion, liquid chromatography-mass spectrometry (LC–MS) tandem analysis, database retrieval analysis, and bioinformatics analysis. The MS raw data were analyzed using DIA-NN (v1.8.1) with the library-free method. The DIA data were reanalysed using this spectral library to obtain protein quantification. After standardization, protein differences were quantitatively analysed using FC and t-test, and the corresponding P values were calculated. FC ≥ 2 or FC ≤ 0.5, VIP > 1, and P-value ≤ 0.05 were defined as significantly different proteins.
Metabolomics analysis
Metabolites were separated by an ultra-performance liquid chromatograph (LC-30A, Shimadzu, Japan) and identified by a mass spectrometer (TripleTOF 6600 + , SCIEX, USA). Peaks with a missing rate > 50% were excluded, with blank values imputed by the KNN method, and peak area was corrected using the SVR method. An Agilent 8890 gas chromatograph coupled to a 5977B mass spectrometer with a DB-5MS column (J&W Scientific, USA) was employed for GC–MS analysis of saccharides.
Unsupervised principal component analysis (PCA) was performed by the statistics function prcomp. The hierarchical cluster analysis results of samples and metabolites are presented as heatmaps with dendrograms, and Pearson correlation coefficients between samples were calculated. Significantly regulated metabolites between groups were determined by the FC. The identified metabolites were annotated using the KEGG compound database (http://www.kegg.jp/kegg/compound/), and the annotated metabolites were then mapped to the KEGG Pathway database (http://www.kegg.jp/kegg/pathway.html).
Statistical analysis
The data were processed and analysed using GraphPad Prism v.8.0.2 (GraphPad). For the comparison of two datasets, Student’s t-test was used. In comparative analyses involving multiple groups, one-way ANOVA was employed, followed by Tukey’s multiple comparisons test to assess significant differences among datasets. The Mantel test was used to evaluate correlations between omics and between omics and outcome measures, with a correlation coefficient ≥ 0.4 and a P-value < 0.05 considered a significant correlation. Data are presented as mean ± standard deviation unless otherwise specified, and a P-value < 0.05 was set to determine statistical significance.

