Cloning, expression, purification, and characterization
To enable the production of a bispecific HER2×VEGF DARPin, we designed a modular construct by fusing a high-affinity anti-HER2 (domain IV) DARPin G3 [21] and an anti-VEGF DARPin Abicipar pegol [23] through a flexible (G₄S)₃ linker, incorporating an N-terminal 6×His-tag-TEV (tobacco etch virus) protease cleavage site and a C-terminal single cysteine residue to facilitate downstream site-specific conjugation (Fig. 1a–b). Both the anti-HER2 DARPin (G3) and anti-VEGF DARPin (Abicipar pegol) are derived from a consensus-designed ankyrin repeat scaffold, originally based on structural motifs found in human ankyrin proteins [21, 25]. This design contributes to their low immunogenicity, favorable folding, and high affinity [21, 23, 25, 26]. The bispecific construct (~ 30 kDa), which incorporates these two domains, maintains the same scaffold architecture and is expected to exhibit comparable immunological and biophysical properties. Note, biparatopic DARPins such as 6L1G (i.e., DARPin 9.26-linker-G3) and 9L1H (i.e., DARPin 9.29-linker-G3) [27,28,29], which simultaneously engage HER2 subdomains I and IV via a short glycine-serine linker (G₄S), have demonstrated potent HER2 signaling inhibition [28]. While these constructs offer a strong structural basis for dual epitope engagement [28, 29], in this study we selected the monospecific anti-HER2 domain IV DARPin G3 for fusion with the anti-VEGF DARPin Abicipar pegol as a proof-of-concept. Monospecific anti-HER2 and anti-VEGF DARPins were also similarly cloned into pET28a(+) vectors for comparative studies (Figure S1 a–f). The bispecific and monospecific DARPins were successfully transformed into E. coli BL21(DE3) cells (Figure S2, Steps 1–2) and recombinant expression was induced using isopropyl β-D-1-thiogalactopyranoside (IPTG). Soluble expression was achieved under standard conditions, with the majority of the DARPin proteins recovered in the supernatant following bacterial lysis and centrifugation (Figure S2, Steps 3–5). Yields of the bispecific DARPin were highly reproducible across independent batches, averaging approximately 100–130 mg/L (Table S1).
DARPin purification was performed using nickel–nitrilotriacetic acid (Ni-NTA) affinity chromatography, exploiting the designed N-terminal 6xHis-tag, followed by size-exclusion chromatography (SEC) to achieve high monomeric purity (Figure S2, Steps 6–7). SEC profiles showed a predominant monomeric peak for the HER2×VEGF bispecific DARPin conjugate at approximately 30.1 kDa, with monospecific DARPins eluting as expected at ~ 15.9 kDa (Fig. 1c). Purified proteins were further characterized by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), confirming expected molecular weights and the successful removal of major contaminants (Fig. 1d). As expected, minor dimer formation was detected among all three types of DARPins expressed, consistent with intermolecular disulfide bond formation. Under non-reducing conditions, the HER2×VEGF DARPin exhibited 1.7% dimer content after SEC purification, which was further reduced to 0.2% and zero following treatment with 1 mM tris(2-carboxyethyl)phosphine (TCEP) or β-mercaptoethanol (BME), respectively (Table S2, Fig. 1d). Monospecific anti-HER2 and anti-VEGF DARPins displayed slightly higher initial dimerization (7.0% and 7.6%, respectively), which was similarly reversible upon reducing agent treatment (Table S2). To evaluate scalability, we performed a 10-fold scale-up from a 30 mL culture (1× scale) to a 300 mL culture (10× scale) (Figure S3a). SDS-PAGE analysis under non-reducing and reducing conditions confirmed that the scale-up did not adversely affect protein purity or integrity (Figure S3b). Densitometry analysis revealed comparable monomer content and minimal dimer formation (1.9% vs. 1.6%), supporting the robustness of the expression and purification processes at larger scales.
It is worth noting that to facilitate optional tag removal, we engineered our construct with an N-terminal 6×His-tag followed by a TEV protease cleavage site (Fig. 1b, black arrowhead). This design allows for efficient and scarless removal of the His-tag when necessary. While the His-tag was retained for functional studies presented herein, including cell binding assays using flow cytometry and enzyme-linked immunosorbent assay (ELISA), cleavage protocols and purification strategies supporting workflows for tag-free proteins are also detailed (see Methods section). Specifically, post-cleavage purification can be efficiently achieved using the similar Ni-NTA affinity chromatography, leveraging the His-tag on TEV protease to separate it and the cleaved tag from the target protein. Furthermore, to ensure suitability for downstream biological applications, all DARPin samples underwent standard endotoxin removal using high-capacity spin columns, and were confirmed to have endotoxin levels below the assay’s detection limit of 0.5 endotoxin units (EU)/mL, as detailed in the Methods section.
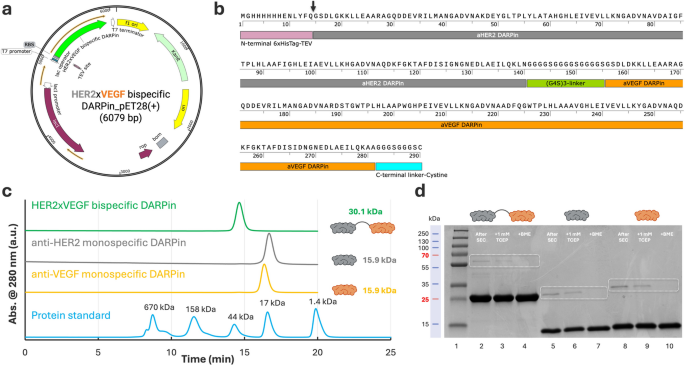
Cloning, expression, and purification of bispecific HER2×VEGF DARPin bioconjugates. (a) Schematic map of the pET28a(+) expression vector containing the HER2×VEGF bispecific DARPin gene (6079 bp) under the T7 promoter, with an N-terminal 6×His-tag and TEV protease cleavage site for affinity purification, and a C-terminal (G3S)2 linker followed by a cysteine residue for site-specific conjugation. (b) Annotated amino acid sequence of the expressed HER2×VEGF bispecific DARPin, showing the N-terminal 6×His-tag and TEV cleavage sequence (pink), anti-HER2 DARPin domain (gray), flexible (G₄S)₃ linker (green), anti-VEGF DARPin domain (orange), and C-terminal cysteine residue (blue). The TEV cleavage site is marked by a black arrowhead. (c) SEC profiles of the purified HER2×VEGF bispecific DARPin (green) and monospecific anti-HER2 (gray) and anti-VEGF (orange) DARPins, demonstrating a predominant monomeric species, compared against protein molecular weight standards (blue). (d) SDS-PAGE analysis of purified DARPins showing expected molecular weights with ~ 30.1 kDa for bispecific HER2×VEGF DARPin, ~ 15.9 kDa for monospecific anti-HER2 and anti-VEGF DARPins), and evaluation before and after reducing treatments with 1 mM TCEP or BME
To further assess the biochemical stability of the HER2×VEGF bispecific DARPin-cysteine conjugate, we performed sequential SEC analysis over a 21-day time-course at both 4 °C and 25 °C (Figure S4, Table S3). Storage at 4 °C maintained a predominant monomeric population, with monomer content decreasing slightly from ~ 100% on Day 0 to 83.9% on Day 21, accompanied by a modest increase in dimer formation from nearly zero to 16.1%. In contrast, samples stored at 25 °C exhibited accelerated dimerization, with monomer content decreasing to 59.5% and dimer increasing to 40.5% by Day 21 (Table S3). Importantly, treatment with 1 mM TCEP at Day 21 effectively restored the DARPin to > 99% monomeric species under both storage conditions, confirming that the observed dimerization was mediated by reversible disulfide bond formation. To further validate these findings, we analyzed the same samples by non-reducing and reducing SDS-PAGE (Figure S5). Densitometry analysis showed that dimer content at Day 21 was 20.8% for samples stored at 4 °C and 37.6% for samples stored at 25 °C, closely matching the SEC quantification (Table S3). Under reducing conditions, all samples reverted predominantly to monomeric forms, further supporting the disulfide-mediated mechanism of dimerization.
To complement these stability assessments, thermal denaturation of the HER2×VEGF bispecific DARPin-cysteine conjugate was evaluated using circular dichroism (CD) spectroscopy at 222 nm, a wavelength sensitive to α-helical content [26]. The unfolding curve displayed a sharp and cooperative transition with a melting temperature (Tm) of approximately 74.5 °C (Figure S6), consistent with a two-state folding model [26, 30]. A slight gradual decline in ellipticity was observed from 20 °C to ~ 70 °C, reflecting subtle pre-transition structural fluctuations, as reported for other thermostable ankyrin repeat proteins [31]. These data confirm the high thermal stability and conformational integrity of the DARPin scaffold, supporting its robustness for site-specific conjugation and advanced biomedical applications.
These results collectively demonstrate that the HER2×VEGF bispecific DARPin-cysteine conjugate possesses good storage stability, particularly under refrigerated conditions, and that any dimerization is largely reversible by mild reduction. Overall, the HER2×VEGF bispecific DARPin demonstrated excellent recombinant expression efficiency, high purity after dual-step purification, and strong biochemical stability, providing a robust platform for subsequent bioconjugation and functional evaluation.
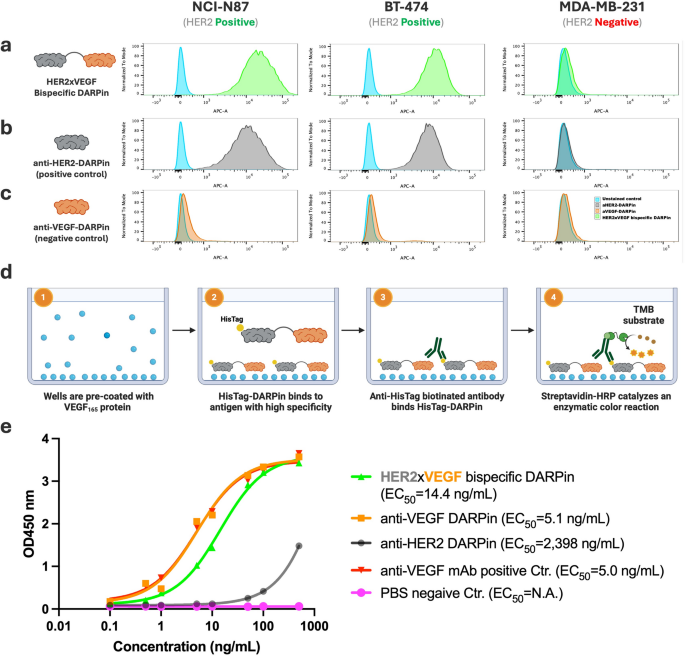
Functional evaluation of HER2×VEGF bispecific and monospecific DARPin binding activities by flow cytometry and ELISA. (a–c) Flow cytometry analysis of DARPin binding to HER2-positive (NCI-N87, BT-474) and HER2-negative (MDA-MB-231) breast cancer cell lines. Cells were incubated with (a) HER2×VEGF bispecific DARPin, (b) anti-HER2 monospecific DARPin (positive control), or (c) anti-VEGF monospecific DARPin (negative control). Binding was detected via anti-HisTag APC-conjugated antibody. (d) Schematic illustration of the anti-VEGF ELISA assay setup. (1) Wells are pre-coated with recombinant human VEGF165 protein. (2) His-tagged DARPins (HER2×VEGF bispecific or monospecific controls) bind specifically to the immobilized VEGF165 antigen. (3) Biotinylated anti-HisTag antibody recognizes the bound DARPins, followed by (4) streptavidin-HRP binding and catalysis of a colorimetric reaction using TMB substrate for detection. (e) ELISA results showing concentration-dependent binding of HER2×VEGF bispecific DARPin and anti-VEGF monospecific DARPin to VEGF165, but no detectable binding by the anti-HER2 monospecific DARPin. Commercial anti-VEGF mAb-biotin and phosphate-buffered saline (PBS) were selected to serve as positive and negative controls for assay validation. EC50 values were determined by nonlinear regression analysis
HER2/VEGF targeted binding assay of DARPins
To confirm the retention of dual-targeting capability in the designed HER2×VEGF bispecific DARPin, we evaluated binding to both HER2 and VEGF using established cell-based and ELISA assays. Monospecific anti-HER2 and anti-VEGF DARPins were used as positive controls, along with commercially available anti-HER2 and anti-VEGF monoclonal antibodies. First, we selected human NCI-N87 gastric and BT-474 breast cancer cell lines, which are widely used HER2-positive models, and MDA-MB-231 as a HER2-negative human triple negative breast cancer control line. The HER2 expression status of these cell lines was validated by flow cytometry using a commercially available anti-HER2 PE-conjugated monoclonal antibody (PE-mAb). As expected, NCI-N87 and BT-474 exhibited strong HER2-specific staining, while MDA-MB-231 showed minimal HER2 expression (Figure S7a–c).
We then assessed HER2-binding activity of the DARPin constructs by flow cytometry using an APC-conjugated anti-HisTag monoclonal antibody to detect DARPin binding. Cells were incubated with 10 nM of either the bispecific DARPin or the respective monospecific DARPins (anti-HER2 or anti-VEGF) at 4 °C for 30 min to allow binding. The HER2×VEGF bispecific DARPin showed specific binding to HER2-positive NCI-N87 and BT-474 cells, with no detectable binding to HER2-negative MDA-MB-231 cells (Fig. 2a). Similarly, the anti-HER2 monospecific DARPin showed selective binding to HER2-positive cells (Fig. 2b), whereas the anti-VEGF monospecific DARPin exhibited no binding to any of the tested cell lines (Fig. 2c), consistent with the absence of VEGF surface expression on these cell lines.
In parallel, we developed a VEGF-specific ELISA assay to assess VEGF-binding capability. Wells were pre-coated with recombinant human VEGF165 protein, and DARPin binding was detected using biotinylated anti-HisTag antibody, followed by streptavidin-horseradish peroxidase (HRP) and 3,3′,5,5′-tetramethylbenzidine (TMB) colorimetric detection (Fig. 2d). The HER2×VEGF bispecific DARPin demonstrated concentration-dependent binding to VEGF165 (EC50 = 14.4 ng/mL), comparable to the anti-VEGF monospecific DARPin (EC50 = 5.1 ng/mL), while the anti-HER2 monospecific DARPin showed no clear VEGF binding (EC50 > 2000 ng/mL) (Fig. 2e). Commercial anti-VEGF mAb-biotin and PBS served as positive and negative controls, respectively, validating the assay specificity. Together, these results confirm that the engineered HER2×VEGF bispecific DARPin retains specific binding to both HER2 and VEGF targets, with comparable performance to monospecific DARPin controls.
Site-specific azide modification of HER2×VEGF bispecific DARPins
To enable downstream site-specific conjugation to nanodelivery vehicles and improve biochemical stability, we selectively modified the free thiol group of the C-terminal cysteine on the HER2×VEGF bispecific DARPin using an Azide-PEG3-Maleimide linker. The thiol–maleimide reaction efficiently introduced an azide functionality to the DARPin, generating the HER2×VEGF bispecific DARPin-azide construct (Fig. 3a). Following conjugation, SEC purification was performed to isolate the monomeric DARPin-azide product and remove unreacted linkers or minor byproducts. The final HER2×VEGF bispecific DARPin-azide conjugate achieved a monomeric purity greater than 98% as assessed by SEC-high-performance liquid chromatography (HPLC) analysis (Figure S8), indicating successful modification and excellent product homogeneity.
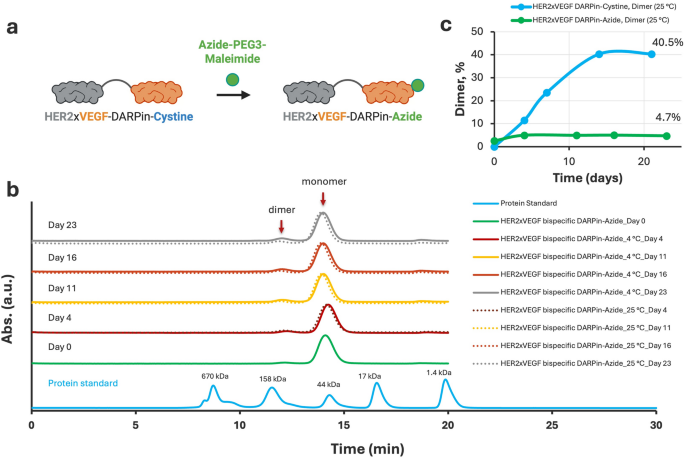
Site-specific modification of HER2×VEGF bispecific DARPin-Cysteine with Azide-PEG₃-Maleimide and comparison of stability profiles before and after conjugation. (a) Schematic illustration of the conjugation strategy: the free thiol group of the C-terminal cysteine in the HER2×VEGF bispecific DARPin-cysteine conjugate was selectively modified with azide-PEG3-maleimide through a thiol–maleimide coupling reaction, generating a HER2×VEGF bispecific DARPin-azide conjugate. (b) SEC profiles monitoring the stability of HER2×VEGF bispecific DARPin-azide conjugates over 23 days at 4 °C (solid lines) and 25 °C (dotted lines). (c) Quantitative comparison of dimer content during storage at 25 °C, highlighting the substantial suppression of dimerization following Azide-PEG3 modification, in contrast to the progressive dimer accumulation seen with the unmodified DARPin-cysteine construct
The stability of the HER2×VEGF bispecific DARPin-azide construct was then evaluated over a 23-day period at both 4 °C and 25 °C (Fig. 3b). In contrast to the parent HER2×VEGF bispecific DARPin-cysteine conjugate, which showed progressive dimerization during storage (Figure S4), the azide-modified DARPin conjugate demonstrated remarkable biochemical stability, with minimal dimer formation, even under elevated temperature conditions. Quantitative analysis of dimer content at 25 °C revealed a striking difference: while the unmodified DARPin-cysteine accumulated up to 40.5% dimer content by Day 21 (Figure S4), the DARPin-azide conjugate maintained less than 5% dimer even after 23 days at 25 °C (Fig. 3c). This significant suppression of dimerization is attributed to the successful blocking of the reactive C-terminal cysteine via thiol–maleimide chemistry, preventing disulfide-mediated intermolecular crosslinking. Overall, these results confirm that site-specific azide-PEG3-maleimide modification not only enables orthogonal click chemistry conjugation for future biomedical and translational applications, but dramatically enhances the biochemical stability and homogeneity of the HER2×VEGF bispecific DARPin under storage conditions.
Proof-of-Concept bioconjugation of HER2×VEGF bispecific DARPin for nanobiotechnology applications
To expand the utility of the engineered HER2×VEGF bispecific DARPin, we demonstrated its compatibility with orthogonal click chemistry-mediated conjugations. Specifically, the azide-functionalized HER2×VEGF bispecific DARPin (HER2×VEGF DARPin-azide) enabled efficient strain-promoted azide–alkyne cycloaddition (SPAAC) reactions with various dibenzocyclooctyne (DBCO)-functionalized moieties, including fluorescent dyes, radiometal chelators, drug-linkers, and nanoparticle platforms (Scheme 1, Table S4). This modular conjugation approach offers a versatile platform for biomedical applications, including, but not limited to, optical imaging, positron emission tomography (PET) imaging, targeted radionuclide therapy, and advanced nanodrug delivery strategies.
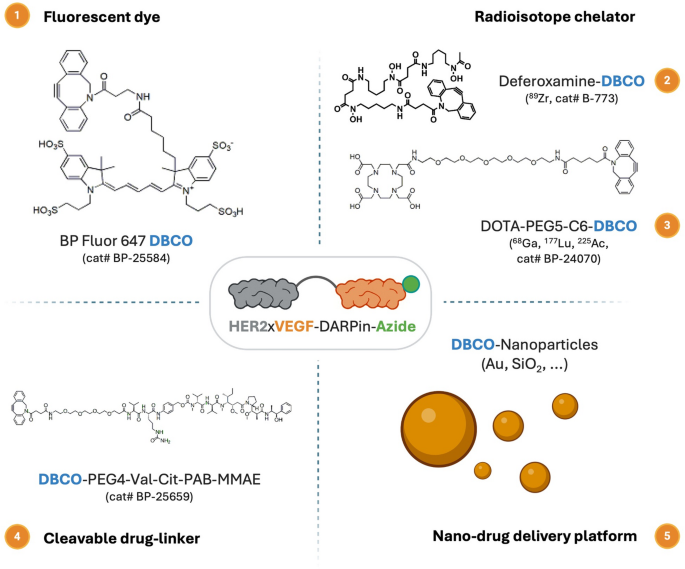
Schematic overview of potential applications for HER2×VEGF bispecific DARPin-azide bioconjugates through orthogonal click chemistry bioconjugation. The azide-functionalized HER2×VEGF bispecific DARPin bioconjugate can undergo SPAAC click chemistry reactions with diverse DBCO-modified payloads, including (1) fluorescent dyes for optical imaging (e.g., BP Fluor 647-DBCO), (2) radioisotope chelators for PET/SPECT (single photon emission computed tomography) imaging or radiotherapy (e.g., deferoxamine-DBCO and DOTA-PEG5-C6-DBCO), (3) cleavable cytotoxic drug linkers (e.g., DBCO-PEG4-Val-Cit-PAB-MMAE) for targeted therapy, and (4) DBCO-functionalized nanoparticles (e.g., Au, SiO2 nanoparticles) for advanced nanomedicine delivery platforms
The successful site-specific conjugation of HER2×VEGF bispecific DARPin-azide to diverse DBCO-functionalized moieties—including fluorophores, chelators, and a cleavable MMAE drug linker—was validated by both SEC-HPLC and SDS-PAGE analyses. SEC-HPLC profiles (Fig. 4) demonstrated distinct left-shifted elution peaks for all conjugated DARPins compared to the unmodified DARPin-azide and DARPin-cysteine controls, indicating increased apparent molecular weight consistent with successful covalent coupling. All conjugates show dominant monomeric purity. A minor dimer peak (gray arrowhead) consistently observed across all profiles likely originates from a small fraction (< 2%) of unconjugated HER2×VEGF DARPin-cysteine (Figure S8), which was retained in all bioconjugated samples. Complementary SDS-PAGE analysis under non-reducing and reducing conditions (Figure S9) further supported the generation of well-defined, high-purity conjugates. Each functionalized variant displayed a clean, expected banding pattern with no evidence of degradation or side-product formation, underscoring the robustness and orthogonality of the click chemistry strategy. Figure S10 further demonstrates efficient and specific bioconjugation of BP Fluor 647 to the HER2×VEGF bispecific DARPin, as confirmed by co-elution of absorbance peaks at both 280 nm and 651 nm during SEC-HPLC dual-wavelength analysis. These data collectively demonstrate that the C-terminal azide-engineered HER2×VEGF bispecific DARPin serves as a versatile scaffold for generating homogeneous, high-purity conjugates suitable for diverse biomedical applications.
SPR assay development and validation of monospecific DARPins
To accurately characterize the bispecific binding behavior of HER2×VEGF DARPins and assess the impact of site-specific bioconjugation, we first established and validated a surface plasmon resonance (SPR) assay using monospecific DARPin controls. This step ensured that the immobilized HER2 and VEGF165 antigens were functionally active and selectively recognized by their respective DARPins. Recombinant human HER2 or VEGF165 proteins were immobilized independently on carboxymethylated dextran sensor chip (CM5) via standard amine coupling, and analytes were injected at concentrations ranging from 0.16 to 160 nM. The binding data were analyzed using a 1:1 Langmuir binding model to extract kinetic parameters (kon, koff) and equilibrium dissociation constants (KD).
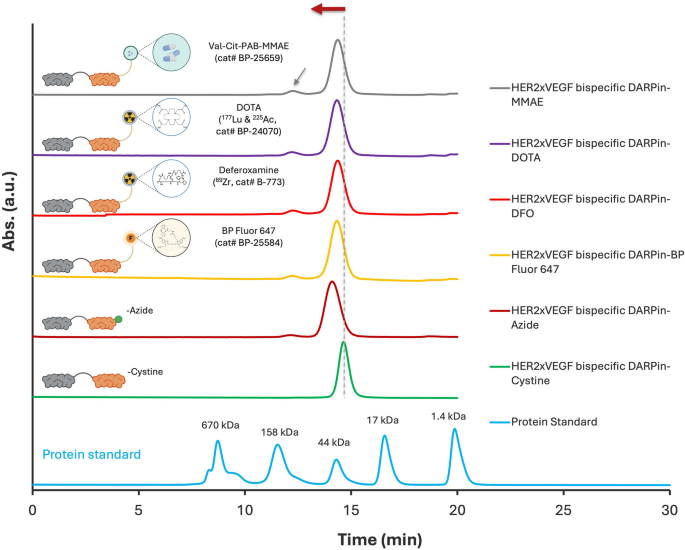
SEC-HPLC analysis confirming successful conjugation of HER2×VEGF bispecific DARPin-Azide to different DBCO-functionalized moieties. SEC-HPLC chromatograms demonstrating efficient bioconjugation to (top to bottom): cleavable MMAE drug linker, DOTA chelator, deferoxamine chelator, BP Fluor 647 dye, compared to the unmodified DARPin-Azide and DARPin-Cysteine precursors. All conjugates show dominant monomeric purity. A minor dimer peak (gray arrowhead) likely derived from residual unconjugated DARPin-Cysteine was consistently present across all bioconjugated samples
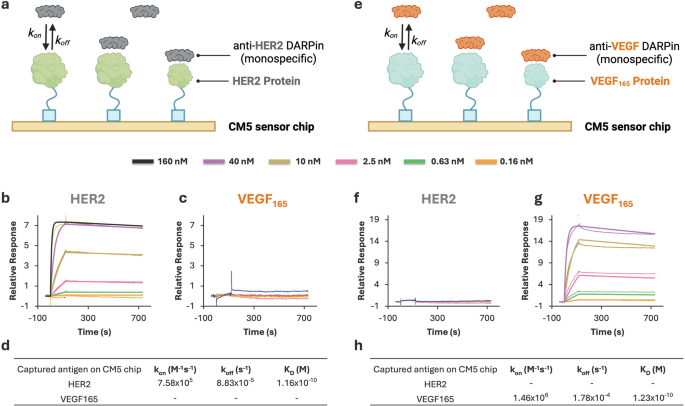
SPR assay development and validation for monospecific DARPins. (a) Schematic of SPR setup with HER2 antigen immobilized on a CM5 chip and anti-HER2 DARPin as the analyte. (b–c) Sensorgrams showing binding of anti-HER2 DARPin to HER2-immobilized chip (b) and no detectable interaction with VEGF165-immobilized chip (c). (d) Kinetic parameters (kon, koff, and KD) for anti-HER2 DARPin interaction with HER2. (e) Schematic of SPR setup with VEGF165 antigen immobilized on a CM5 chip and anti-VEGF DARPin as the analyte. (f–g) Sensorgrams showing no detectable binding of anti-VEGF DARPin to HER2-immobilized chip (f) and specific interaction with VEGF165-immobilized chip (g). (h) Kinetic parameters (kon, koff, and KD) for anti-VEGF DARPin interaction with VEGF165
As shown in Fig. 5, the anti-HER2 monospecific DARPin construct exhibited strong, specific binding to the HER2-coated surface (KD= 1.16 × 10−10 M, or 116 pM), with no detectable interaction on VEGF165-coated chips. Conversely, the anti-VEGF DARPin construct bound exclusively to VEGF165 (KD = 1.23 × 10−10 M, or 123 pM) and showed no response on HER2-immobilized surfaces. These results confirm the specificity of the DARPins and validate that each chip surface was appropriately prepared and functionally selective.
This assay validation provides a robust foundation for subsequent kinetic analyses of HER2×VEGF bispecific DARPin conjugates. By immobilizing HER2 and VEGF165 antigens separately, we can resolve and quantify binding to each target independently. This platform enables precise assessment of bispecific binding behavior in both unmodified and bioconjugated DARPins and supports direct comparisons of binding affinity and kinetics across a variety of functional modifications. In all subsequent studies, kinetic constants were extracted using the validated 1:1 Langmuir model, ensuring consistent and reproducible analysis of binding interactions.
Dual antigen targeting by functional HER2×VEGF bispecific DARPin bioconjugates
To evaluate the dual-targeting capability of HER2×VEGF bispecific DARPin conjugates engineered with modular click-reactive sites, we performed SPR assays using the validated CM5 sensor chips with independently immobilized HER2 or VEGF165 antigens (Fig. 6a). Following the assay development strategy established earlier, we characterized binding responses of several conjugated DARPin variants functionalized for molecular imaging or therapeutic use, including fluorophores, radiometal chelators, and cytotoxic drug-linkers (Fig. 6b–m). Representative kinetic constants are summarized in Table 1.
We first confirmed the high-affinity binding of the unmodified HER2×VEGF DARPin-cysteine conjugate and its site-specifically modified derivative, a DARPin-azide conjugate. Both constructs demonstrated dual-target engagement with picomolar-to-subnanomolar equilibrium dissociation constants: KD= 77.4 pM for HER2 and 338 pM for VEGF165 for the DARPin-cysteine construct. These values represent potent antigen engagement, in line with reported high-affinity DARPins against HER2 and VEGF [23, 25]. Importantly, the binding affinities of both DARPin constructs compared favorably with those of commercially available biotinylated monoclonal antibodies, namely anti-HER2 antibody (cat# BAF1129, KD = 524 pM) and anti-VEGF antibody (cat# BAF293, KD = 52 pM) (Table 1). Notably, sensorgrams for these two constructs displayed extremely slow dissociation phases (Figure S11 c–g), a known limitation in SPR analysis of very strong binders such as DARPins [32]. These observations reinforce the robustness of the paratope architecture, even after modular cysteine-azide substitution.
Subsequent SPR analyses of functionally modified HER2×VEGF bispecific DARPins—including conjugates bearing BP Fluor 647 dye, DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid), deferoxamine (DFO), and a cleavable MMAE linker—demonstrated preserved binding to both HER2 and VEGF165, with equilibrium dissociation constants (KD) ranging from approximately 1 to 3 nM (Table 1). Compared to the parent DARPin-cysteine construct, the conjugates retained comparable association rates (kon), while exhibiting modestly faster dissociation rates (koff), accounting for the slight reduction in overall affinity. Despite this shift, all constructs maintained clinically relevant low-nanomolar binding capacity for both targets. These results indicate that site-specific click-based functionalization at the engineered distal cysteine site imposes minimal structural or steric disruption to the antigen-binding interface. This kinetic trend is consistent with prior studies of engineered DARPins, such as PEGylated and dye-labeled variants targeting epithelial cell adhesion molecule (EpCAM) [33], which also showed preserved specificity and only minor changes in functional affinity following bioconjugation. Together, these findings support the robustness of our HER2×VEGF bispecific DARPin scaffold and validate its modular architecture as a versatile platform for targeted imaging and therapeutic applications.

