Detection of nucleotide mutations
Due to the limited availability of long RNA samples with defined mutations, we first validated RNA-SCAN using MS2 RNA as a model carrier scaffold (Supplementary Fig. 2) and synthetic nanolatches carrying specific base changes (Fig. 2a). Specifically, we designed six nanolatch variants targeting the MS2 RNA site, each carrying a distinct sequence alteration: three with single-nucleotide substitutions at different positions (MHm1–MHm3), one with two substitutions (MHm4), one with an insertion (MHm5) and one with a deletion (MHm6). Each nanolatch was incubated separately with the carrier at a tenfold stoichiometric excess (Supplementary Fig. 3). The resulting constructs were analysed using nanopore measurements.
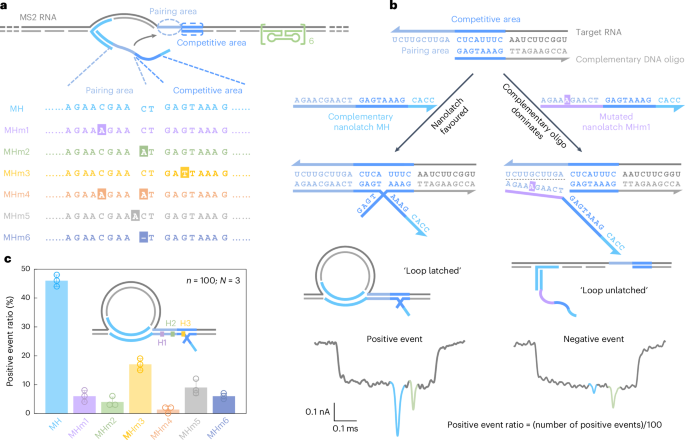
a, A sketch of the MS2 carrier design and sequence comparison between the complementary nanolatch (MH) and its six mutated variants (MHm1–MHm6), highlighting nucleotide changes in both pairing and competitive areas that interact with the target site on the MS2 carrier. b, The mechanism of the competitive interaction between the MS2 RNA target site and nanolatches/complementary DNA oligo, illustrated using the fully complementary nanolatch MH and single-nucleotide mutated nanolatch MHm1. The representative nanopore current traces exhibit distinct signatures corresponding to the ‘loop latched’ (positive event) and ‘loop unlatched’ (negative event) states. The positive event ratio is defined as the number of positive events divided by the total number of events per measurement (n = 100). c, An analysis of positive event ratios for nanolatches MH–MHm6. The bar charts present positive event ratios from three independent measurements (N = 3) for each nanolatch. The data are shown as the mean ± standard deviation.
Source data
The sensitivity of RNA-SCAN to single-nucleotide variations stems from the finely tuned competitive hybridization mechanism at the target site (Fig. 2b). Specifically, each target site is engineered with a 10-nt pairing area and an adjacent 8-nt competitive area. When assembling the carrier, the competitive area of the RNA scaffold is complemented by a DNA oligo, while the pairing area is intentionally left single-stranded to allow full nanolatch binding. This strategic design establishes a delicate competition where the target RNA can transiently hybridize with either the nanolatch or the complementary DNA oligo. When the RNA sequence fully matches the nanolatch, their interaction is thermodynamically favourable, yielding latched loop structures, which manifest as characteristic spike signatures in nanopore measurements (Fig. 2b, left). However, even a single-nucleotide mismatch weakens the nanolatch-RNA binding affinity, tipping the balance in favour of the complementary oligo, which forms a more stable duplex with the RNA scaffold. This competitive displacement of nanolatch increases the likelihood of an unlatched state, characterized by the absence of the large current spike associated with the latched configuration (Fig. 2b, right). We classify translocation events as ‘positive’ when the characteristic spike is present and ‘negative’ when it is absent. A current threshold of 0.55 (Extended Data Fig. 1) was applied to facilitate classification. The positive event ratio is defined as the number of positive events out of 100 total recorded events per nanopore measurement. Optimization of the pairing area and competitive area is shown in Extended Data Fig. 3.
Figure 2c shows the positive event ratios calculated from the first 100 linear nanopore events (n = 100) in each experiment, with error bars from three independent repeats (N = 3). This standardized quantification ensures reliable yet efficient analysis across conditions (Supplementary Fig. 4). When the nanolatch sequence was fully complementary to the MS2 RNA target site (MH), the positive event ratio approached 50%. This level reflects the dynamic nature of loop formation and represents a balance optimized for single-nucleotide sensitivity. In the presence of a single-nucleotide mismatch, the positive event ratio consistently dropped, though the magnitude of reduction depended on the mutation’s position. Mutations in the pairing area (MHm1 and MHm2) led to an approximate tenfold decrease, with positive event ratios falling to ~5%, while the change in the competitive area (MHm3) resulted in a higher average ratio of ~17%. This is probably because mutations in the competitive area primarily affect the competition between the nanolatch and the complementary oligo without disrupting the initial nanolatch-RNA pairing, thereby allowing loop formation to proceed more readily. To ensure high sensitivity and specificity of RNA-SCAN, we strategically positioned mutated or chemically modified nucleotides within the pairing area in subsequent designs. Two mismatches (MHm4) further suppressed loop formation, almost approaching the detection limit. Nucleotide insertion (MHm5) or deletion (MHm6) in the nanolatch also disrupted its interaction with the carrier, reducing the positive event ratio to below 10%. These results demonstrate that RNA-SCAN is highly sensitive to subtle sequence variations and can discriminate single-base differences through easily interpretable nanopore signatures (Extended Data Fig. 4).
Detection of nucleotide modifications
Having demonstrated the ability of RNA-SCAN to detect single-nucleotide mutations, we next applied this methodology to epigenetic nucleotide modifications, which play a critical role in many fundamental metabolic functions3,4.
We first examined 5-methylcytosine (denoted as 5mC in DNA and m5C in RNA), a key epigenetic marker frequently observed in cancer21,22. To investigate its presence and abundance in nucleic acids, we evaluated the RNA-SCAN system from two complementary directions. First, the covalent introduction of a methyl group to cytosine alters the van der Waals radii around the C5 position and modifies base stacking potential. Prior studies have shown that such methylations can enhance duplex stability by influencing thermodynamic properties23,24. Alternatively, bisulfite treatment can selectively deaminate unmethylated cytosines to uracil while leaving 5-methylcytosines intact25,26. This chemical conversion alters the base-pairing preference of cytosine from guanine to adenine, thereby disrupting hybridization patterns (Fig. 3a). Following these principles, we designed five nanolatch variants containing 0–4 5mC modifications and measured loop formation with and without bisulfite conversion. The nanopore measurement results are summarized in Fig. 3b. Without bisulfite conversion, increasing the number of 5mC modifications led to higher positive event ratios, reaching ~69% for the four-modification nanolatch, which is nearly 1.5 times the ratio observed with the unmodified control (~48%). With bisulfite conversion, the positive event ratio for the four-methylated nanolatch remained relatively high at ~52%, whereas nanolatches containing fewer 5mC modifications exhibited a substantial decrease in positive events. This pattern suggests that bisulfite-induced deamination of unmethylated cytosines disrupted their pairing with guanines, destabilizing loop formation and leading to unlatching.
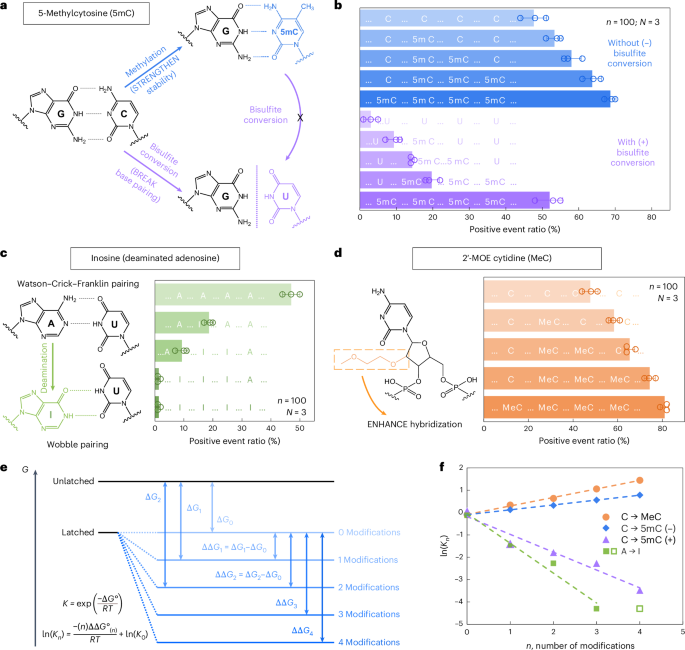
a, A schematic depicting how 5mC modification influences C/G base pairing before and after bisulfite conversion. The MS2 carrier design is identical to that in Fig. 2. b, The nanopore measurement results of MS2 carriers incubated with nanolatches containing 0–4 5mC modifications, with (+) and without (−) bisulfite treatment. c, A schematic of how inosine modification affects A/U base pairing, along with nanopore measurement results for nanolatches containing 0–4 inosines in the sequence. d, A schematic of MeC modification and nanopore measurement results for nanolatches possessing 0–4 MeCs. The data in b–d are shown as the mean ± standard deviation from three independent measurements. e, A theoretical model of the nanolatch system for detecting modifications. The presence of modifications can either strengthen or weaken the stability of the duplex between the nanolatch and the RNA scaffold, altering the energy difference \(\Delta G\) between ‘latched’ and ‘unlatched’ energy levels. R is the ideal gas constant, T is the absolute temperature at which the reaction occurs, \({K}_{n}\) is the equilibrium constant of loop latching, and \(n\) is the number of modifications. f, The linear fitting results of \(\mathrm{ln}({K}_{n})\) against \(n\) for each type of modifications studied in this work. 5mC (+) and 5mC (−) refer to cytosine methylations with (+) and without (−) bisulfite treatment, respectively. The data point for four inosines (open square) was excluded from fitting, as the positive event ratio had already dropped to nearly 0% with three inosines.
Source data
We next tested the ability of RNA-SCAN to detect inosine, a product of adenosine deamination that is associated with RNA editing and post-transcriptional gene regulation27,28. Chemically, this amino-to-keto conversion alters the base’s hydrogen bonding properties, transforming adenosine from a hydrogen bond donor into an acceptor. As a result, canonical Watson–Crick–Franklin base pairing with uridine is replaced by two weaker, non-canonical wobble hydrogen bonds29,30 (Fig. 3c, left). This change destabilizes the RNA/DNA duplex and alters loop formation within the carrier. To evaluate this effect, we designed five nanolatches containing 0–4 inosine substitutions. Nanopore measurements (Fig. 3c, right) showed that even a single inosine substitution reduced the positive event ratio from ~47% to ~19%. With three inosines present, loop formation was almost completely disrupted, and the signal dropped to near zero. These results confirm that RNA-SCAN can sensitively detect the structural consequences of inosine incorporation.
To assess RNA-SCAN’s ability to detect sugar modifications, we incorporated 2′-O-methoxyethyl (2′-MOE) groups into cytidines (2′-MOE cytidine, MeC), which are known to enhance duplex stability in antisense oligo therapeutics31,32 (Fig. 3d, left). Substituting 5mC with MeC in nanolatches led to even greater positive signals (Fig. 3d, right): four MeC modifications yielded ~81% positive events, compared with ~69% with 5mC and ~48% with unmodified sequences. Even a single MeC increased signal by over 10%, highlighting this modification’s strong stabilizing effect on base pairing.
To interpret these findings, we modelled loop formation as a two-state energy system: latched versus unlatched (Fig. 3e). Modifications shift the energy of the latched state, altering its equilibrium. Based on the nearest-neighbour model33,34, which assumes additive energetic contributions from adjacent base pairs, we observed strong linear correlations between the number of modifications and the equilibrium constant (Fig. 3f). These trends aligned with our experimental results and support the thermodynamic basis for modification detection. Further details are available in Supplementary Note 2.
Discrimination of E. coli and S. Typhi by RNA-SCAN
To evaluate the performance of RNA-SCAN on naturally occurring long RNA, we applied it to differentiate between Escherichia coli and Salmonella enterica serovar Typhi (S. Typhi), two closely related Gram-negative bacteria of clinical relevance. While most E. coli strains are harmless gut commensals, some pathogenic variants cause severe gastrointestinal illness35,36. S. Typhi, a human-specific pathogen, is responsible for enteric (typhoid) fever and presents distinct antimicrobial resistance profiles compared with E. coli37,38.
To enable species-level discrimination, we focused on 16S ribosomal RNA (rRNA), a highly conserved but diagnostically informative bacterial identity marker. Sequence alignment across all 14 operons from both species revealed >99% intraspecies similarity and 97–98% interspecies similarity (Supplementary Fig. 5). Within this context, we identified an 18-nt region containing a consistent single-nucleotide difference between E. coli and S. Typhi 16S rRNA and selected it as the target for RNA-SCAN detection (Fig. 4a,b). Separate oligo pools were designed to assemble RNA carriers from the total 16S rRNA of each species, while nanolatches were tailored to match the species-specific variant of the target sequence. Each carrier was incubated with either a matched or mismatched nanolatch, creating four experimental combinations.
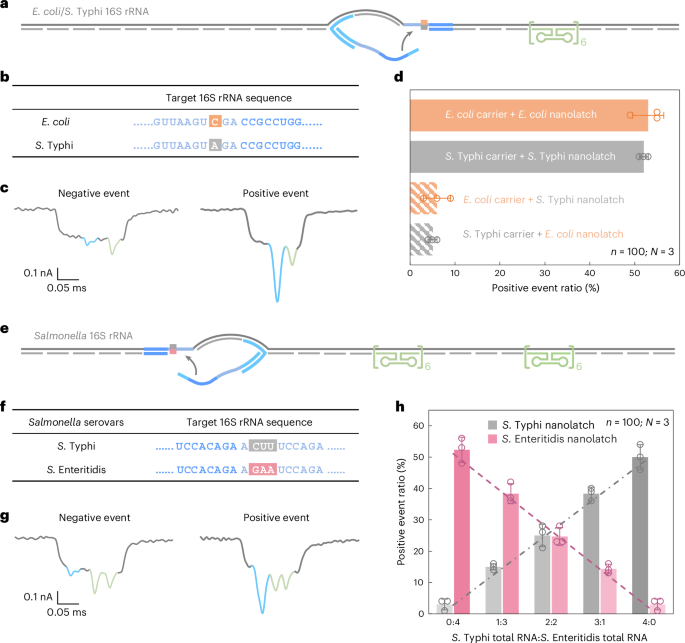
a, A schematic of the E. coli/S. Typhi 16S rRNA carrier design with a reference structure positioned on one side of the carrier and a target site located at the centre. b, The sequences of E. coli and S. Typhi 16S rRNA at the target site, highlighting a single-nucleotide variation. c, Representative nanopore current traces of the E. coli/S. Typhi 16S rRNA carrier. d, The analysis of the positive event ratio for the four combinations of E. coli/S. Typhi carrier with E. coli/S. Typhi nanolatch. The carrier concentration was set at 0.3 nM at the start of nanopore measurements and the nanolatch concentration at 3 nM for both E. coli and S. Typhi. e, A schematic of the Salmonella 16S rRNA carrier design with two reference structures placed on one side and a target site on the other side. f, The sequences of S. Typhi and S. Enteritidis 16S rRNA at the target site, highlighting three continuous different bases. g, Representative nanopore current traces of the Salmonella 16S rRNA carrier. h, An analysis of the positive event ratio for total RNA mixtures of S. Typhi and S. Enteritidis at defined input ratios, using either the S. Typhi or S. Enteritidis nanolatch. The combined concentration of S. Typhi and S. Enteritidis 16S rRNA carriers was maintained at 0.3 nM in all nanopore measurements, with a constant nanolatch concentration of 3 nM. The data in d and h are shown as mean ± standard deviation from three independent measurements.
Source data
Nanopore measurements were conducted using total RNA directly extracted from E. coli and S. Typhi cultures, without additional purification or enrichment. In matched combinations—E. coli carrier with E. coli nanolatch or S. Typhi carrier with S. Typhi nanolatch—the positive event ratio exceeded 50%, indicating successful loop formation (Fig. 4d). By contrast, the mismatched combinations yielded positive event ratios of around 5%, demonstrating a clear loss of nanolatch binding. This nearly tenfold difference in signal underscores the high sensitivity and specificity of RNA-SCAN in distinguishing single-nucleotide differences within long, native RNA molecules (Extended Data Fig. 5). Importantly, the assay requires no enzymatic processing, amplification or fragmentation and works directly with total bacterial RNA, making it highly suitable for practical applications such as microbial identification and resistance profiling.
Quantification of Salmonella 16S rRNA variants
While the high sequence similarity of 16S rRNA between E. coli and S. Typhi already poses a challenge for conventional assays, different serovars of Salmonella enterica, such as S. Typhi and S. Enteritidis39, are even more difficult to distinguish, with 16S rRNA sequence identities exceeding 99.5% overlap (Supplementary Fig. 6).
To address this, we designed a shared oligo pool that binds conserved regions in both S. Typhi and S. Enteritidis 16S rRNA, using consensus nucleotides at polymorphic sites. The carrier design included two reference structures on one side to distinguish this construct from earlier E. coli/S. Typhi carriers (Fig. 4e and Extended Data Fig. 6) and a nanolatch binding site on the other side where three consecutive nucleotides differ between the two serovars (Fig. 4f). To mimic clinical conditions where RNA from multiple bacterial species may coexist, we mixed total RNA extracted from S. Typhi and S. Enteritidis in defined ratios. After hybridizing the mixture with the oligo pool to generate carriers for both serovars, each mixture was split and incubated with either a S. Typhi-specific or S. Enteritidis-specific nanolatch.
The nanopore measurements showed that the positive event ratio increased linearly with the proportion of each target RNA in the mixture when probed with its matching nanolatch (Fig. 4h). This linear relationship confirms that RNA-SCAN can not only distinguish between highly similar sequences but also quantify their relative abundance in complex RNA backgrounds. The small error bars and consistent fits further indicate that other minor sequence variations or intraspecies polymorphisms did not interfere with detection. These results highlight the potential of RNA-SCAN for multiplexed pathogen quantification in mixed samples, with single-nucleotide resolution and without requiring prior sample purification or amplification.
m5C detection in 16S rRNA from E. coli and A. baumannii
Beyond sequence variation, we further evaluated the ability of RNA-SCAN ability to detect base modifications in endogenous long RNA. In E. coli, rRNAs contain at least 24 known methylation sites essential for ribosomal function40. Among these, three are m5C modifications located at positions C967 and C1407 in 16S rRNA (Fig. 5a) and at C1962 in 23S rRNA. Notably, methylation at C1407 is highly conserved in E. coli and has been implicated in translation fidelity40,41. By contrast, Acinetobacter baumannii, a common cause of healthcare-associated opportunistic infections, completely lacks the methyltransferase gene responsible for C1407 methylation42,43. This makes E. coli and A. baumannii ideal comparative models for studying RNA methylation at this position.
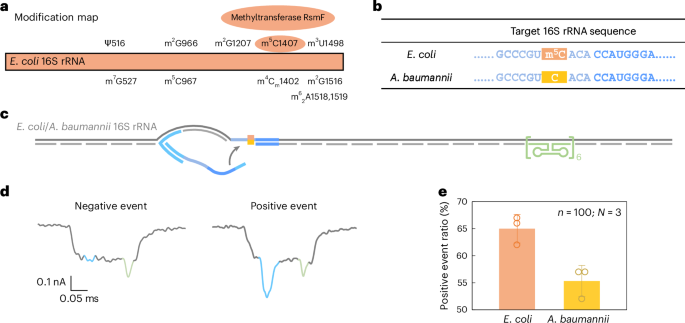
a, A modification map of E. coli 16S rRNA, highlighting the essential role of the methyltransferase RsmF in catalysing methylation at cytosine 1407. b, The sequences of E. coli and A. baumannii 16S rRNA at the target site. The cytosine at position 1407 is methylated in E. coli 16S rRNA but remains unmethylated in A. baumannii 16S rRNA. c, A schematic of the E. coli/A. baumannii 16S rRNA carrier design, featuring a reference structure on one side of the carrier and the target site at the opposite end. d, Representative nanopore current traces of the E. coli/A. baumannii 16S rRNA carrier. e, The analysis of the positive event ratio for E. coli and A. baumannii 16S rRNA carriers using the same nanolatch. The carrier concentration was maintained at 0.3 nM and the nanolatch concentration at 3 nM. The higher positive event ratio observed for E. coli can be attributed to the stabilizing effect of the m5C modification at the target site. The data are shown as mean ± standard deviation from three independent measurements.
Source data
Given the substantial sequence divergence of over 15% between their 16S rRNA operons (Supplementary Fig. 7), we designed species-specific oligo pools for assembling RNA carriers, while using a common nanolatch targeting the C1407 region. The resulting carrier structure included the C1407 target site on one side and a reference structure on the other (Fig. 5b,c). The total RNA extracted directly from bacterial cells was used without further processing.
Nanopore measurements showed a statistically significant difference in loop formation between species. E. coli produced a positive event ratio of 65% ± 2%, compared with 55% ± 2% for A. baumannii (Fig. 5e and Extended Data Fig. 7), with a t-test yielding P = 0.01289. These results are consistent with our earlier MS2-based experiments, supporting the interpretation that m5C1407 enhances nanolatch binding by stabilizing C/G base pairing. Interestingly, both species exhibited higher signal levels than unmodified MS2 carriers. To investigate this, we performed bisulfite sequencing on the 16S rRNA target region. The results (Extended Data Fig. 8) suggest that another nearby modification, m4Cm1402 (a 2′-O-methylated and N4-methylated cytosine found in both species), is partly resistant to bisulfite conversion and may similarly strengthen base pairing.
Together, these findings demonstrate the capability of RNA-SCAN to detect single-base RNA modifications in native, unamplified samples. Moreover, the observation of enhanced signals from nearby modified nucleotides suggests that RNA-SCAN could also serve as a tool for identifying previously unannotated modifications in functionally important RNA regions.

