Synthesis and characterization of PDA@AgNP nanozyme
PDA stabilizes AgNPs against aggregation and oxidation through catechol/amine-mediated anchoring, while its π-π stacking and hydrogen bonding enhance colloidal stability. Redox-active catechol groups further quench radicals and synergize with AgNP catalysis, boosting oxidative stability and adaptive catalytic performance. These properties make PDA-AgNP composites highly effective for practical applications [39]. PDA is self-assembled in an alkaline environment from dopamine, followed by the deposition of AgNP through one-step reduction (Fig. S1). Under scanning electron microscope (SEM), PDA self-assembly is noted, and the gray represents AgNP indicating successful synthesis of PDA@AgNP (Fig. 1a and Fig. S2). For clearer visualization, transmission electron microscopy (TEM) confirmed abundant AgNP (Fig. 1b and S3). After attaching AgNP, the average particle size of PDA@AgNP slightly increased (Fig. S4). Energy dispersive spectrometry (EDS) of PDA@AgNP revealed that the total mass fraction of silver was 17.7% (Fig. S5), and the inclusion resulted in changes in zeta potential (Fig. S6) and UV-vis absorption (Fig. S7) showcasing the successful AgNP deposition. The integration of PDA and AgNPs enhances PCE by accelerating charge transfer [40], consistent with observed superior photothermal performance of PDA@AgNP over PDA alone in aqueous environments (Fig. S8).
These results indicate successful PDA@AgNP fabrication, and the addition of AgNP significantly enhances PCE, providing foundation for efficient biofilm disruption. To fully utilize the potential of PDA@AgNP, incorporating it into hydrogels prevents PDA self-aggregation [41].
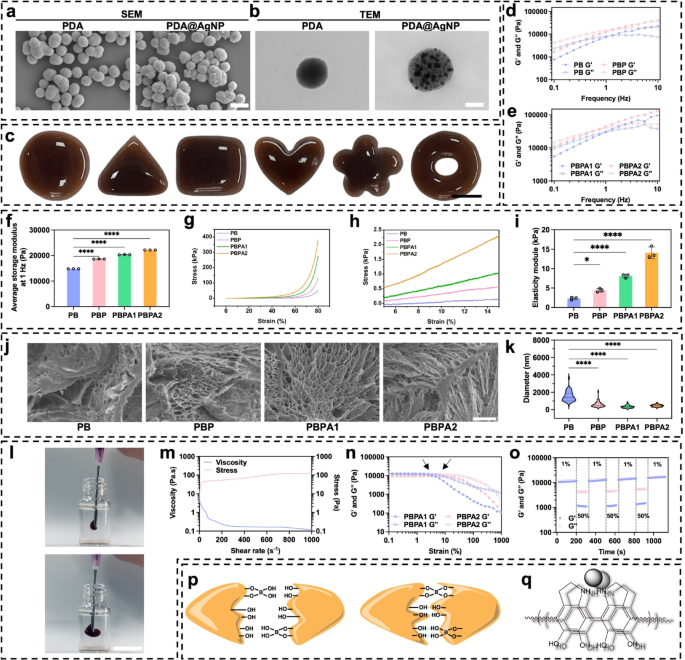
Characterization, and mechanical properties of PDA@Ag and PBPA hydrogel. a) SEM of PDA and PDA@AgNP, scale bar = 400 nm; b) TEM of PDA and PDA@AgNP, scale bar = 110 nm; c) Plasticity of PBPA2 hydrogel, scale bar = 1 cm; d) Frequency sweep test (from 0.1 to 10 Hz) at 37 °C of PB and PBP; e) Frequency sweep test (from 0.1 to 10 Hz) at 37 °C of PBPA1 and PBPA2; f) Quantitative average storage modulus at 1 Hz of the hydrogels, n = 3; g) Compression test with stress-strain curve from 0% to 80% of different hydrogel; h) strain from 5% to 15% of compression test of different hydrogel; i) Elasticity modulus of different hydrogels in the 5–15% strain range of compression curves, n = 3; j) SEM images of different hydrogels, scale bar = 5 μm; k) The pore size of the hydrogel with different components, n = 50. l) injection of PBPA2 hydrogel, scale bar = 1 cm; m) viscosity measurements of PBPA2 with increasing shear rate at a fixed frequency of 1 Hz under continues stress; n) Rheological properties of the hydrogel in strain amplitude sweep (γ = 0.1–1000%) at 1 Hz; o) Rheological property of PBPA2 at low-1% and high-50% strains; p) Dynamic hydrogen and B-O bond of PBPA; q) π-π stacking of PDA@AgNP. Data represented mean ± SD. **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05
Fabrication, characterization, and mechanical properties of PBPA hydrogel
PVA, valued for its biocompatibility and biodegradability [42], was crosslinked via dynamic BEB to introduce ROS responsiveness [43]. Borax-mediated BEB formation between PVA diol groups and PDA@AgNP (Fig. S9) yields a hydrogel stable in neutral to slightly alkaline conditions yet degradable under acidic, ROS-rich wound environments [44]. ROS-triggered BEB cleavage enables controlled nanozyme release [45, 46].
Fourier transform infrared spectroscopy (FTIR) confirmed successful BEB crosslinking, indicated by the peak at 1334 cm−1 (Fig. S10). To probe component-specific roles, hydrogels with varying compositions were prepared: PB (PVA-BEB-PVA), PBP (PVA-BEB-PDA), PBPA1 (PVA-BEB-1 unit PDA@ AgNP), and PBPA2 (2 units). Enhanced BEB crosslinking was observed with PDA presence and increasing PDA@AgNP content, evidenced by elevated intensities at 1334 cm−1 and 1660 cm−1 (C = O stretch) (Fig. S11) [47], which was highest in PBPA2 due to the increased amount of PDA.
Hydrogels containing different components were successfully synthesized (Fig. S12) with excellent plasticity (Fig. 1c), allowing them to be personalized to wound shapes. This exceptional plasticity originates from the distinctive viscoelastic properties exhibited by PVA and BEB (Fig. 1d and e), allowing the material to flow like a liquid yet retain shape after yielding. The average G’ at 1 Hz increased when addition of BEB and PDA@AgNP (Fig. 1f), due to additional crosslinking introduced by catechol groups from PDA [45, 46]. PDA enhances energy dissipation, increasing both G′ and G′′. AgNPs act as physical crosslinking sites, uniformly dispersed to restrict polymer chain mobility and further augment energy dissipation via interfacial interactions [47]. This synergy significantly improves mechanical properties, reflected in the highest compressive strength at 80% deformation for PBPA2 (Fig. 1g), likely due to strongest crosslinking. Additional reinforcement arises from hydrogen bonding and π-π stacking between PDA and PVA [48]. Elasticity modulus increases significantly which also attributed to these reasons (Fig. 1h and i). Critically, prolonged exposure to high modulus (>50 kPa) induce mechanical memory in fibroblasts, promoting myofibroblast activation and sustained fibrosis [49]. Specifically, low modulus and degradability avoid initiating this mechanical stress-myofibroblast activation-collagen synthesis feedback loop. By preventing persistent myofibroblast activity, the system supports scar-inhibited regenerative healing, addressing a key challenge in wound treatment [49]. Despite the strongest crosslinking, the elastic module of PBPA2 is less than 20 kPa (Fig. 1i). Moreover, each hydrogel maintained high stretchability, extending nearly tenfold (Fig. S13 and S14). The strong plasticity is likely because of the freeze-casting, allowing PVA to form micro-scale honeycomb-like pore walls consisting of interconnected nanofibril meshes (Fig. 1j and S15) [50]. The pore size of the PVA hydrogel decreases with dopamine addition (Fig. 1k), primarily due to increased crosslinking from hydrogen bonding, BEB, and π-π interactions. Introduction of PDA enhanced network compactness, while further incorporation of PDA@AgNP tightened the structure via pseudocrosslinking mediated by nanoparticle–polymer dynamic interactions [51]. Doubling PDA@AgNP concentration elevated crosslinking density, promoting further densification. Despite reduced pore size, injectability was maintained. The hydrogel exhibited shear-thinning behavior under increasing shear rates (Fig. 1l and m), enabled by reversible BEB breakage [44, 52]. This was enhanced by PVA viscoelasticity and PDA interactions. Rapid post-injection structural recovery was facilitated by hydrogen bonding and π-π stacking through PDA, supporting self-healing (Fig. S16). Incorporation of AgNPs further enhanced self-healing by re-establishing conductive networks upon material rejoining enabling recovery within seconds [53]. After complete rupture under shear (Fig. 1n), the hydrogel rapidly self-healed upon force removal (Fig. 1o and S17). As mentioned above, these mechanical properties were mainly because of BEB, hydrogen bonds, and π-π stacking (Fig. 1p and q).
While the hydrogel’s skin-compatible modulus facilitates wound application, its relatively low mechanical strength may compromise stability. Rheological evaluation under varied conditions showed retained viscoelasticity after one month in sealed storage (Fig. S18). However, rigidity increased after one day in dry air, likely due to water loss. In moist conditions simulating wound exudate, both G′ and G′′ decreased markedly, suggesting swelling-induced structural expansion and partial matrix degradation. After NIR irradiation, hydrogel rigidity increased significantly. Photothermal conversion by AgNP and PDA elevated local temperature, enhancing chain mobility and promoting reorganization of dynamic BEB into a denser network. Hydrogen bonding and BEB stability also improved, further increasing crosslinking density. Elevated temperature may additionally induce polymer rearrangement or phase transition, leading to network compaction [54, 55]. These mechanisms collectively account for the rise in G′ post-irradiation.
In summary, PBPA exhibits remarkable plasticity, tensile strength, self-healing properties, and injectability, which satisfy its use in various wounds. Its viscoelastic nature ensures conformal skin coverage, while rapid self-healing withstands repeated mechanical stress at joint sites. The low module, stable across various conditions, avoids healing suppression and minimizes local tension. These superior mechanical properties give hydrogel strong foundation for wound application.
Adhesiveness, hemostasis, and swelling ability of PBPA hydrogel
The PBPA hydrogel exhibits strong adhesion through multiple mechanisms (Fig. 2a). PDA and PVA provide abundant hydroxyl and amino groups that form hydrogen bonds with polar skin residues [56, 57]. Dynamic BEB react reversibly with skin hydroxyl groups, offering robust yet adaptive adhesion. Although AgNPs do not directly contribute to adhesion, their antibacterial action helps maintain interfacial cleanliness, supporting sustained adherence. Furthermore, the micro-rough surface structure from directional freezing (Fig. 1j), enhancing physical interlocking. The hydrogel maintains firm adhesion during joint flexion and fully recovers after bending at various angles (Fig. 2b and c), indicating its suitability for covering skin, especially on frequently bent joints. To assess its adhesiveness, the hydrogel was evenly applied between two pieces of porcine skin (Fig. S19 and 2f). Stretching tests revealed a significant increase in adhesiveness upon the addition of PDA, with PBPA2 showing the best performance, characterized by the longest stretch distance and highest tensile stress (Fig. 2d), along with increased adhesive strength (Fig. 2e). This exceptional adhesive performance was also observed in other organs and materials (Fig. S20 and S21).
The PBPA hydrogel demonstrated effective hemostatic capacity in a rat liver bleeding model (Fig. 2g), significantly reducing total blood loss within two minutes (Fig. 2h). This effect is likely attributable to its strong tissue adhesion and rapid fluid absorption, which help remove exudate, inhibit eschar formation, and eliminate microbial niches, thereby supporting subsequent regeneration [1]. The hydrogel maintained water absorption for up to 24 h (Fig. 2i). PBPA2 showed the highest swelling ratio both initially (Fig. 2j) and at 24 h (Fig. 2k), while retaining structural integrity (Fig. 2i), likely due to its higher PDA content and compact pore structure. Additionally, PBPA2 exhibited superior water retention, preserving ~ 50% absorbed water after one week (Fig. S22), owing to its dense, dual-crosslinked porous network [58]. This moisture-locking capability prevents desiccation, sustains a moist healing environment, and minimizes syneresis, ensuring consistent wound contact [59].
The PBPA hydrogel achieves rapid hemostasis and efficiently absorbs wound exudate, key drivers of eschar formation. By eliminating these factors, it suppresses eschar development while maintaining a moist, conforming wound interface. This hydrated, eschar-free microenvironment facilitates cell migration and re-epithelialization, providing ideal conditions for regeneration and promoting healing with reduced scarring.
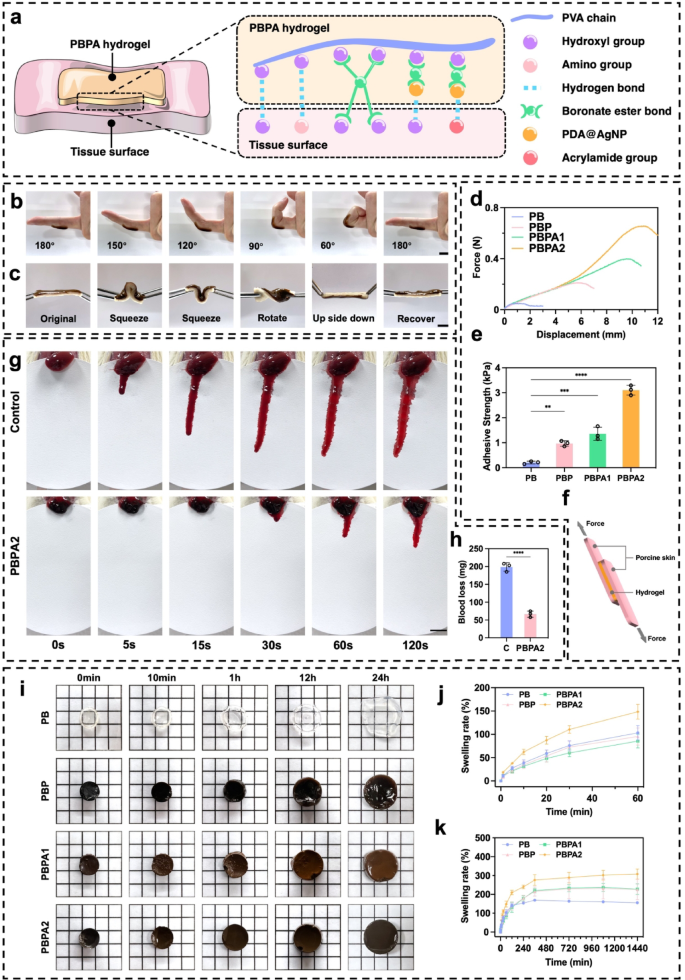
Adhesiveness, hemostatic, fluid absorption, and swelling ability of the hydrogel. a) Possible adhesive mechanisms of PBPA hydrogel to tissue. b) Application of PBPA2 hydrogel when bending joint, scale bar = 1 cm; c) Application of PBPA2 hydrogel on porcine skin, scale bar = 1 cm; d) Force-displacement curves of different hydrogels-bonded porcine skin; e) Quantitative adhesive strength of the hydrogels calculated from the force-displacement curves, n = 3; f) Schematic figure of the lap shear adhesive test using porcine skin and the hydrogel; g) Representative images of the hemostatic test using rat liver, scale bar = 1 cm; h) Quantitative blood loss, n = 3; i) Representative images of different hydrogels emerged in PBS at different time points, size of small square = 5 mm*5 mm; j) Quantitative swelling analysis of different hydrogels from 0–60 min, n = 3; k) Quantitative swelling analysis of different hydrogels from 0–24 h, n = 3; Data represented mean ± SD. **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05
In vitro validation of ROS-responsive nanozyme delivery and ROS-scavenger properties of PBPA hydrogel
The PBPA hydrogel dynamically regulates ROS through BEB-triggered nanozyme release and PDA-mediated scavenging, achieving homeostasis to simultaneously enhance antibacterial action and support tissue regeneration (Fig. 3a). H2O2 was used to simulate the effect of high ROS environments [60]. In an environment with 2mL 500 µM H2O2, the 300 µL hydrogels degraded rapidly within 10 h (Fig. 3b), with PBPA2 exhibiting the slowest degradation, due to its higher degree of crosslinking mentioned previously. In contrast, in simulated low ROS environments, such as PBS (Fig. S23) or simulated wound environments containing lysozyme (Fig. 3c), confirming its H2O2-responsive release behavior. This results from oxidation-induced loss of electrophilicity at the boron center under high H2O2, disrupting BEB stability and triggering nanozyme release [61, 62]. To verify the release of PDA@AgNP from the hydrogel, absorption peak at 576 nm was identified through UV-vis analysis of different PDA@AgNP concentrations (Fig. S24a). A linear regression model was established based on the absorbance values at 576 nm across various concentrations, enabling quantitative concentration determination (Fig. S24b). Notably, the hydrogel demonstrated rapid, H2O2-responsive release of PDA@AgNP nanozymes under high H2O2 conditions (Fig. S24c), consistent with its degradation profile. Despite Fenton-like activity, radical scavenging dominated the therapeutic outcome, as confirmed by DPPH and NBT assays. PBPA2 exhibited the strongest scavenging capacity, evidenced by colorimetric changes (Fig. 3d, S25, and S26), with sustained efficacy over 24 h. This highlights PDA’s superior antioxidant role over AgNP radical generation, enabling dual antibacterial and antioxidant regulation. In vitro experiments using DCFH-DA labeling to assess ROS levels further elucidated the ultimate antioxidant performance in H2O2 induced in vitro model (Fig. 3e). Compared to the H2O2 group, the PB group, which contained BEB but no PDA, already showed ROS depletion, while PDA further enhanced this performance. Quantitative flow cytometry confirmed the same results with DCFH-DA fluorescence (Fig. 3f and S27), further substantiating the ROS responsiveness and scavenging capabilities. Collectively, these results confirm that the intrinsic ROS-scavenging capacity of BEB and PDA remains effective even after incorporation of AgNPs, whose nanozyme-generated ROS does not impair overall antioxidant performance. This establishes a reliable basis for in vivo application.
As local ROS concentrations decrease in a wound, it facilitates cell migration and proliferation in later phases, promoting wound healing. Thus, experiments on keratinocyte migration inhibition by H2O2 with different hydrogels were conducted. H2O2 significantly inhibited migration, with treatment using PBPA1 failing to restore migration to baseline levels. Only the introduction of PBPA2 resulted in migration levels comparable to the negative control (Fig. 3g and h). So, both adequate BEB crosslinking and sufficient PDA are essential for re-epithelialization. PBPA hydrogels significantly mitigated the damage caused by H2O2 to keratinocytes and fibroblasts ensuring their survival and proliferation (Fig. 3i and k). Although other hydrogels were able to reduce H2O2 damage to some extent, they could not achieve the excellent performance exhibited by PBPA2 (Fig. S28).
In summary, the ROS-responsive BEB in PBPA senses infection and triggers on-demand release of antibacterial AgNP nanozymes, which generate Ag+ and radicals. Unlike conventional ion-leaching nanobioceramics, this mechanism avoids long-term metal accumulation and cytotoxicity [63]. Subsequent PDA scavenging further refines ROS management, enabling dual control of bacteria and oxidative stress, a programmed strategy that achieves ROS-balanced therapy.
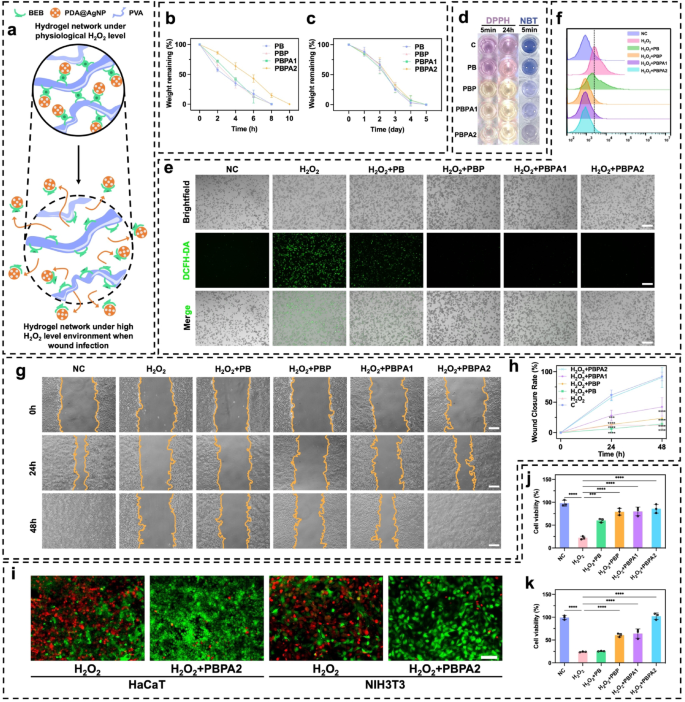
ROS-depend intelligent delivery and ROS-scavenger properties of the hydrogels. a) Schematic image of ROS-depend degradation and cargo-release of the PBPA hydrogel; b) Hydrogel degradation rate in 500 µM H2O2 environment, n = 3; c) Hydrogel degradation rate in 1 mg/ml lysozyme, n = 3; d) Representative images of DPPH and NBT test of different hydrogel; e) Intracellular ROS-scavenger performance of different hydrogels by DCFH-DA test on RAW264.7 cell line under H2O2 stimulation, scale bar = 200 μm; f) Flowcytometry of DCFH-DA labeled RAW264.7 cell in fluorescein isothiocyanate FITC-A channel on different hydrogels; g) Representative images of HaCaT migration under H2O2 environment treating with different hydrogels, scale bar = 100 μm; h) Quantitative analysis of HaCaT migration, comparison was made to C group, n = 3; i) Representative images of live/dead staining of HaCaT and NIH3T3 cell under H2O2 environment treating with or without PBPA2 hydrogel, scale bar = 50 μm; j) Quantitative analysis of live/dead staining of HaCaT under H2O2 environment treating with different hydrogels, n = 3; k) Quantitative analysis of live/dead staining of NIH3T3 under H2O2 environment treating with different hydrogels, n = 3. Data represented mean ± SD. **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05
Photothermal ability, biofilm disruption, and antibacterial effect of PBPA hydrogel
AgNP integration enhances the PCE of PDA, markedly improving biofilm disruption and enabling efficient AgNP delivery. Effective biofilm eradication facilitates the transition from inflammation to proliferation, while localized thermal activity promotes collagen remodeling and accelerates skin regeneration [64]. This constitutes the crucial step in the overall therapeutics.
PB exhibited no photothermal effects under any irradiation (Fig. S29). Upon the addition of PDA, an increase in temperature was observed (Fig. S30). After adding AgNP, the increase in temperature became more pronounced (Fig. S31), consistent with results observed in aqueous systems, confirming the enhanced PCE through AgNP deposition. PBPA2 displayed the strongest photothermal performance (Fig. 4a and b), with superior clear distinctions observed during 1 W/cm² (Fig. S32) and 2 W/cm² (Fig. 4c) NIR irradiation. Furthermore, PBPA2 also demonstrated rapid heating-cooling capabilities, allowing for effective reuse across four cycles within 1 h under both 1 W/cm² and 2 W/cm² NIR irradiation (Fig. S33 and 4 d). PBPA2 exhibited the highest PCE (Fig. 4e), attributable to AgNP enhancement and elevated PDA@AgNP content. It also demonstrated superior thermal stability (Fig. 4f), enabling optimal NIR responsiveness, efficient heat generation, higher peak temperatures, and consistent performance, making it ideal for rapid, repeatable, and stable photothermal biofilm disruption.
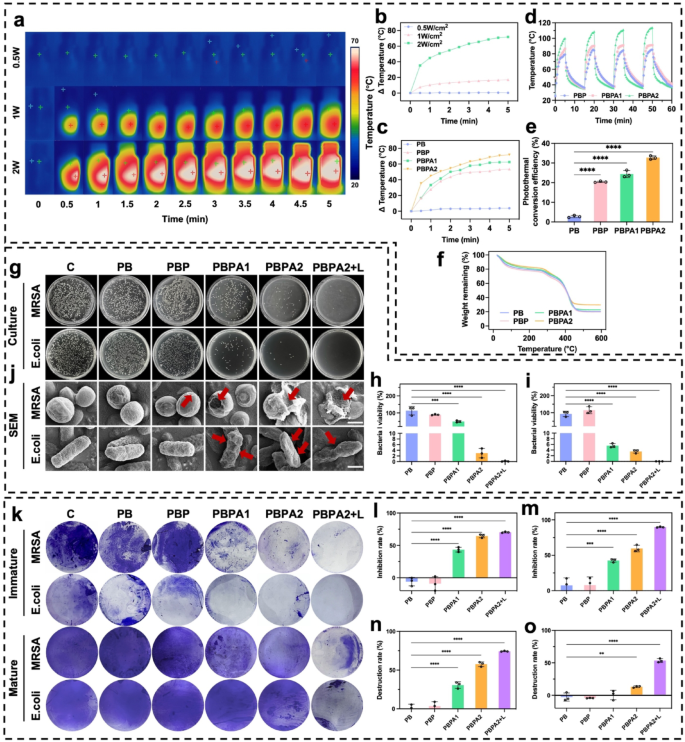
Photothermal effect, artificial triggered biofilm disruption, and antibacterial effect of different hydrogels. a) Representative images of PBPA2 hydrogels under different power of 808 nm NIR; b) Quantitative analysis of the photothermal effect of PBPA2 hydrogels under different power of 808 nm NIR; c) Quantitative analysis of the photothermal effect of different hydrogels under 2 W/cm2 808 nm NIR; d) Photothermal stability under repeated on-and-off 808 nm NIR of different hydrogels; e) PCE of different hydrogels, n = 3; f) Photothermal stability of TGA curves of different hydrogels; g) Representative images of colonization of MRSA and E. coli under different hydrogel treatments; h) Quantitative MRSA bacterial viability from colonization after different hydrogel treatments, n = 3; i) Quantitative E. coli bacterial viability from colonization after different hydrogel treatments, n = 3; j) Representative SEM images of MRSA and E. coli under different hydrogel treatments, scale bar = 500 nm, red arrow indicates the destruction; k) Representative images of the immature biofilm inhibition and mature biofilm destruction under different hydrogel treatments stained by crystal violet; l) Quantitative analysis of immature MRSA biofilm inhibition after different hydrogel treatments, n = 3; m) Quantitative analysis of immature E. coli biofilm inhibition after different hydrogel treatments, n = 3; n) Quantitative analysis of mature E. coli biofilm destruction after different hydrogel treatments, n = 3; o) Quantitative analysis of mature MRSA biofilm destruction after different hydrogel treatments, n = 3. Data represented mean ± SD. **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05
Antibacterial efficacy against Escherichia coli (E. coli) and methicillin-resistant Staphylococcus aureus (MRSA) was assessed via plate culture. AgNP-containing hydrogels alone significantly inhibited bacterial growth (Fig. 4g and i), but the application of photothermal effects led to nearly 100% bacterial elimination. SEM confirmed disruption of bacterial morphology under different hydrogel treatments (Fig. 4j) and bacteria destruction by PDA@AgNP (Fig. S34). The combined antibacterial and ROS-scavenging capabilities of PBPA improve the wound microenvironment, shorten the inflammatory phase, and promote progression to proliferation. While AgNPs effectively inhibit planktonic bacteria and immature biofilms (Fig. 4k and m and S35), NIR-induced photothermal disruption is essential to eradicate mature biofilms (Fig. 4k and S36). In cases biofilms matured, the standalone AgNPs was insufficient for overcoming the biofilm. Despite the ability of PBPA2 to disrupt mature biofilms formed by E. coli (Fig. 4n), its efficacy in treating MRSA biofilms was not evident without NIR exposure (Fig. 4o). NIR irradiation is essential for PBPA2 to disrupt mature biofilms, serving as the critical initial step that enables subsequent therapeutic efficacy.
In summary, PBPA2 exhibits exceptional and reusable photothermal properties, enabling nearly 100% bacterial inhibition and providing a favorable microenvironment. It potently suppresses immature biofilm formation and, under NIR irradiation, robustly disrupts mature biofilms. This photothermal initiation opens therapeutic pathways for subsequent nanozyme delivery, making it highly suitable for practical applications.
In vitro anti-inflammation effect and biocompatibility of PBPA hydrogel
Previous experiments verified that PBPA mitigate oxidative stress abnormalities caused by high concentrations of H2O2 (Fig. 3e). In infected wounds, the pathological microenvironment features high ROS and LPS activity. LPS drives M1 macrophage polarization and excessive ROS generation, which the PBPA hydrogel effectively attenuates, most notably with higher PDA loading (Fig. S37). This suppression is attributed to PDA-derived dopamine and quinone, which mitigate LPS-induced inflammation [65]. LPS-induced ROS overproduction primarily stems from M1 polarization, as shown by elevated CD86 (Fig. 5a and b) and iNOS (Fig. 5a and d) expression [66]. In contrast, M2 polarization marked by elevated CD206 (Fig. 5a and c) expression is significantly suppressed. PBPA treatment effectively reprograms this LPS-driven polarization pattern toward anti-inflammatory M2 phenotypes (Fig. 5a and d). Notably, PDA incorporation not only reverses LPS-induced M1 polarization but also achieves levels comparable to interleukin (IL)−4-induced positive controls (Fig. 5e).
LPS exacerbates inflammation in NIH3T3 fibroblasts [67] and upregulates α-SMA expression in NIH3T3 cells [12], potentiating permanent myofibroblast activation and pathological scarring [49]. Previous studies demonstrate that LPS-stimulated macrophage-fibroblast co-cultures significantly elevate TGF-β expression [13], which serves as the primary upstream regulator of α-SMA. Using conditioned medium from LPS-polarized macrophages (MS) to treat NIH3T3 effectively models this macrophage-driven fibrotic response [66], capturing α-SMA induction via inflammatory-proliferative crosstalk. Key findings reveal that MS-cultured NIH3T3 cells exhibit elevated α-SMA expression (Fig. 5f and g). Consistent with anti-inflammatory effects on macrophages in vitro, PDA significantly attenuates fibrotic responses, with PBPA2 exhibiting the strongest efficacy, confirming that PBPA2 modulates macrophage behavior to downstream suppress α-SMA expression and myofibroblast activation.
Biocompatibility and toxicity are critical for clinical translation. Hemolysis assays confirmed that all hydrogels exhibited hemolysis rates below 5% when cocultured with blood (Fig. 5h). In vitro biocompatibility was assessed using RAW264.7 macrophages, HaCaT keratinocytes, and NIH3T3 fibroblasts. Live/dead staining after 24-hour coculture confirmed good compatibility (Fig. 5i and l and S38). Moreover, five-day continuous exposure to hydrogels induced no significant toxicity (Fig. 5m and o).
The PBPA hydrogel modulates M1 polarization under infection, promoting a shift toward M2 phenotypes to shorten the inflammatory phase. It also suppresses LPS- and M1-induced fibroblast overactivation, reducing fibrotic scarring. Additionally, the hydrogel demonstrates excellent biocompatibility without detectable cytotoxicity in vitro.
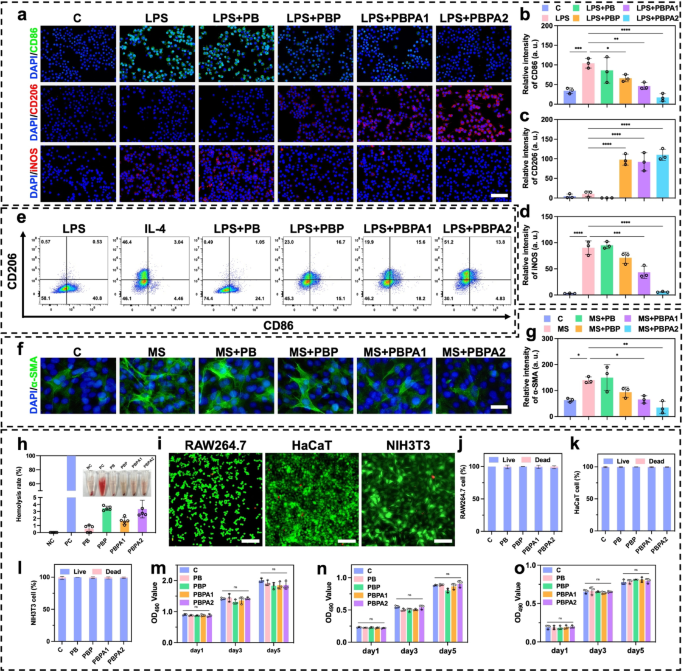
In vitro anti-inflammation effect and biocompatibility of different hydrogels. a) Immunofluorescence staining of CD86, CD206, and iNOS on RAW 264.7 under different treatment, scale bar = 100 μm; b) Relative intensity of CD86 based on immunofluorescence staining, n = 3; c) Relative intensity of CD206 based on immunofluorescence staining, n = 3; d) Relative intensity of iNOS based on immunofluorescence staining, n = 3; e) Flow cytometry analysis of CD206 and CD86 on RAW 264.7 under different treatment; f) Immunofluorescence staining of α-SMA on NIH3T3 under different treatment, scale bar = 100 μm; g) Relative intensity of α-SMA based on immunofluorescence staining, n = 3; h) Quantitative analysis and representative images of the hemolysis test after treating with different hydrogels, n = 3; i) Representative images of live/dead staining of RAW264.7, HaCaT, and NIH3T3 treated with PBPA2 hydrogel, scale bar = 50 μm; j) RAW264.7, k) HaCaT, and l) NIH3T3 live/dead staining quantitative analysis after treating with different hydrogels, n = 3; m) RAW264.7, n) HaCaT, and o) NIH3T3 MTT test quantitative analysis after treating different hydrogels at corresponding time point, n = 3. Data represented mean ± SD. **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05
PBPA accelerating regenerative infected wound healing in vivo
PDA@AgNP achieves a unique balance between antibacterial activity and ROS modulation, unlike metal-oxide nanozymes (e.g., CeO2), which exhibit strong catalysis but limited photothermal efficiency and adaptability. By integrating the peroxidase-like activity of AgNPs with PDA’s antioxidant and photothermal capacities, PDA@AgNP enables concurrent biofilm disruption, bacterial eradication, and ROS clearance. This dual functionality stabilizes AgNPs against oxidation and allows programmable catalytic response, outperforming conventional single-function nanozymes in biofilm-infected wound treatment. To evaluate practical performance, MRSA and E. coli biofilm-infected models were established 48 h prior to treatment. Experiments commenced once a yellowish membrane confirmed biofilm formation (Fig. 6a). Photothermally triggered biofilm disruption achieved the required temperature within just 10 s (Fig. 6b and S39). On D3 of treatment, although wound area showed no significant difference among groups (Fig. 6c), the PBPA2 + L group displayed markedly reduced redness, minimal exudate, and no swelling (Fig. 6d and e), indicating near-complete eradication of biofilm and bacteria, along with successful local ROS consumption, consistent with the ROS-responsive antimicrobial release. This early intervention laid the groundwork for accelerated healing, with PBPA2 + L achieving optimal wound closure rates of 80.27% (D7) and 99.92% (D14) (Fig. 6c and e). Critically, PBPA2 + L significantly reduced early exudation and hemorrhage, limiting eschar formation; by D14, substantial eschar was observed in all other groups except PBPA2 + L (Fig. 6d). Control experiments confirmed that therapeutic benefits originated from photothermal activation rather than NIR irradiation alone (Fig. S40). Bacterial culture of wound exudate on D3 and D7 demonstrated strong antibacterial efficacy (Fig. 6f and g). Additionally, blood tests on D14 showed that the C group still exhibited elevated white blood cell (WBC) levels, indicating ongoing infection (Fig. 6h), mainly characterized by increased neutrophil levels (Fig. 6i and l). Although the PB and PBPA2 groups showed slight reductions in neutrophils, levels remained above normal, underscoring that only PBPA2 + L therapy achieved full therapeutic effectiveness.
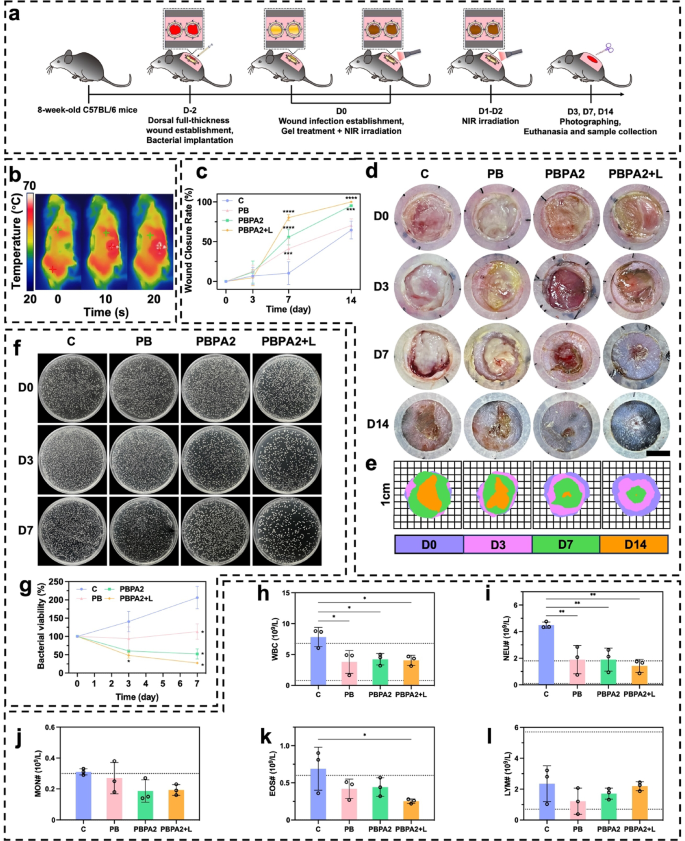
In vivo application of different treatment for biofilm-infected wound. a) Schematic illustration of the procedure for biofilm infection model establishment and programmed treatment. b) Representative infrared images of the photothermal effect of PBPA2 hydrogel on mice; c) Quantitative analysis of wound healing rate under different treatments, n = 3; d) Representative images on different time points of biofilm-infected wounds treated with different treatments, scale bar = 4 mm; e) Schematic images on different time points of biofilm-infected wounds treated with different treatments; f) Representative images of colonization of bacteria on wound exudate at determined time point after different treatments; g) Quantitative analysis of corresponding bacterial colonization, n = 3; h) White blood cell counting of blood samples from mice treated with different treatments, n = 3; i) Neutrophil counting of blood samples from mice treated with different treatments, n = 3; j) Monocyte counting of blood samples from mice treated with different treatments, n = 3; k) Eosinophil counting of blood samples from mice treated with different treatments, n = 3; l) Lymphocyte counting of blood samples from mice treated with different treatments, n = 3. Data represented mean ± SD. **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05
H&E staining was used for histological assessment of wound healing (Fig. 7a). On D7, significant wounds remained in the C and PB groups, whereas hydrogel treatments promoted granulation tissue formation and increased dermal thickness (Fig. 7b and d). However, only under the PBPA2 + L treatment was rapid re-epithelialization on D7 (Fig. 7a), consistent with gross observations (Fig. 6d). These observations suggest that on D7, the C and PB groups remained in the hemostatic-inflammatory phase, whereas groups treated with PBPA2, with or without light, had advanced to the proliferative-remodeling phase. Rapid re-epithelialization, as seen in the PBPA2 + L group, reduces the risk of hypertrophic scarring and supports regenerative healing [64]. By D14, only the C group displayed non-healing wounds with persistent infection and eschar. Although other groups achieved full re-epithelialization (Fig. 7e), but the PB and PBPA2 groups showed significant scarring (Fig. 7c), characterized by reduced local skin appendages and extensive granulation tissue fibrosis. This suggests that PBPA2 + L significantly inhibits scar formation and enhances skin regeneration.
Masson’s staining and fractal analysis revealed more abundant and organized collagen deposition in the PBPA2 + L group by D7 (Fig. 7f and g), indicative of advanced proliferative phase transition and a pro-regenerative fibroblast microenvironment. Quantitatively, programmed treatment led to a more two-dimensional collagen architecture (Fig. 7h) and lower lacunarity (Fig. 7i), reflecting superior matrix organization. However, this result reversed by D14. The C group eventually initiated fibroblast proliferation and collagen secretion through intrinsic healing mechanisms, while treated groups, especially PBPA2 + L, exhibited looser collagen distribution, indicating active remodeling (Fig. 7f). Fractal analysis showed sparser collagen in PBPA2 + L, characteristic of mature remodeling that reduces mechanical tension and creates space for skin appendage regeneration. Quantitatively, PBPA2 + L displayed the lowest collagen density (Fig. 7j). This may be due to fibroblasts secreting a provisional, fibrin-rich ECM rather than a collagen-rich one, thus promoting scarless and rapid wound healing [49]. Additionally, collagen distribution was further far from a two-dimensional structure (Fig. 7k), and lacunarity was higher (Fig. 7l), providing remodeling space for skin appendage regeneration and reducing local tension.
These results suggest that PBPA2 + L treatment advances the progression of the healing phases, shortening the inflammatory phase and rapidly transitioning to the proliferative phase to promote swift wound healing. Furthermore, under the comprehensive management of multi-modal treatments, the local structure becomes more relaxed, reducing tension-induced scar formation and facilitating the shift from a repair mode to a regeneration mode.
Interestingly, differences of healing pattern between PBPA2 + L group and other groups were observed (Fig. 7m), as evidenced by a predominantly peripheral-to-central healing pattern, with less granulation tissue from bottom up, a similar morphology of healing center to the surrounding tissue, and an accelerated re-epithelialization. In contrast, other groups were mostly filled with granulation tissue bottom up. This suggests the treatment shifted healing pattern to “peripheral-to-central” rather than “bottom-to-up”, which provide basis for subsequent remodeling and skin regeneration. To verify this hypothesis, orientation-related analyses is needed. However, there is currently no suitable method in skin. In conjunction with the previously reported scar patterns [68], a new analytical method in following section that spatially divides the skin based on the healing results and analyses each region separately was proposed in the present study to demonstrate that programmed treatment can improve regeneration efficiency by directing healing orientation.
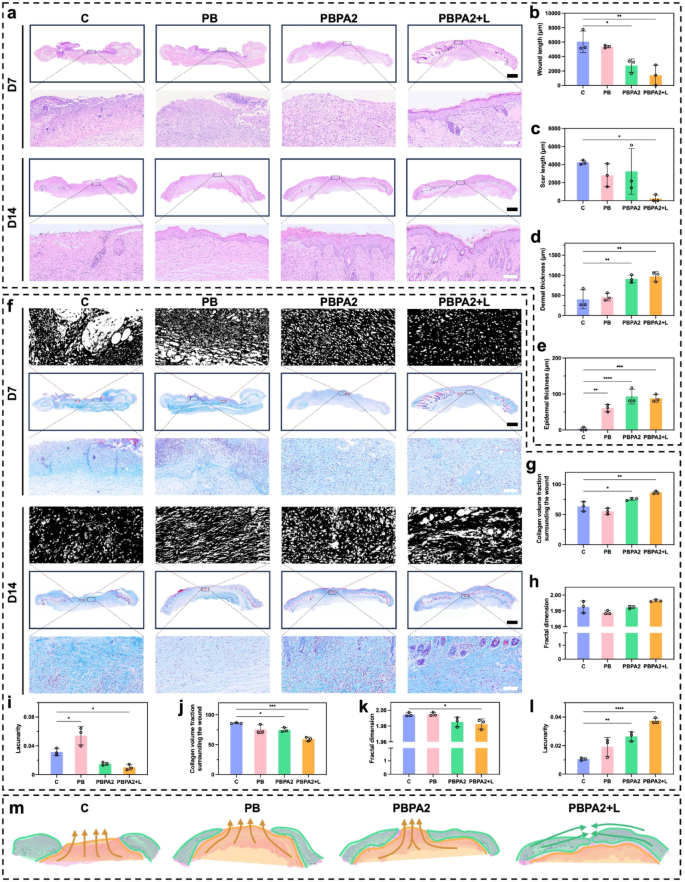
H&E and Masson’s staining of skin section for evaluation of skin regeneration. a) H&E staining of skin on D7 and D14 in different treatment group, scale bar of whole section = 1 mm, scale bar of enlarged part = 100 μm; b) Wound length on D7 calculated through H&E staining, n = 3; c) Scar length on D14 calculated through H&E staining, n = 3; d) Dermal thickness on D7 calculated through H&E staining, n = 3; e) Epidermal thickness on D14 calculated through H&E staining, n = 3; f) Masson’s staining on D7 and D14 in different treatment group with fractal images, scale bar of whole section = 1 mm, scale bar of enlarged part = 100 μm; g) Collagen volume fraction surrounding wounds of D7 calculated through Masson’s staining, n = 3; h) Fractal dimension calculated on wound area of D7 through Masson’s staining, n = 3; i) Lacunarity calculated on wound area of D7 through Masson’s staining, n = 3; j) Collagen volume fraction surrounding scars of D14 calculated through Masson’s staining, n = 3; k) Fractal dimension calculated on scar area of D14 through Masson’s staining, n = 3; l) Lacunarity calculated on scar area of D14 through Masson’s staining, n = 3; m) Healing orientation pattern difference among groups (orange indicates “bottom-to-top mobilized fascia scar filling mode”, green indicates “peripheral-to-central normal tissue creeping repair mode”). Data represented mean ± SD. **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05
Healing orientation changes after the treatment revealed by patch repair division method analysis
Wound healing is a mobilized patch repair of the underlying fascia, resembling a volcanic crater (Fig. 8a) [68]. To better analyze spatial healing patterns, this study introduces the Patch Repair Division Method, which divides the wound-scar/regeneration zone into three distinct regions (Fig. 8a). Superficial Regeneration Area (a): Resulting from centripetal migration of surrounding dermal and epidermal tissues, this region often exhibits near-normal structure with skin appendages and lies within the original wound boundary outside the central scar. Superficial Scar Area (b): Formed by rapid upward closure from underlying fascia and granulation tissue, this central region lacks typical skin architecture and function. Deep Scar Area (c): Originating from deep mobilized fascia that migrates upward, this area forms the base of the central scar. These regions differ fundamentally in location, extent, orientation, and microstructure of repair. To test the hypothesis that programmed treatment alters healing orientation, each region was compared to corresponding normal skin areas alongside directional analysis, enabling precise evaluation of regenerative outcomes.
Across all three regions, the PBPA2 + L group showed the most pronounced green staining, indicating a type III collagen-rich healing pattern (Fig. 8b and e). A reduced type I/III collagen ratio signifies lower scarring risk and reflects distinct ECM remodeling dynamics. Healthy remodeling typically features early type III collagen deposition, later gradually replaced by type I collagen, a key indicator of wound maturity. Unexpectedly, groups with poorer outcomes exhibited higher type I collagen, while the best-healing group (PBPA2 + L) maintained a type III-dominant profile. This suggests that although all groups reached advanced ECM remodeling stages, PBPA2 + L uniquely preserved high type III collagen levels, accelerating healing while minimizing scar formation.
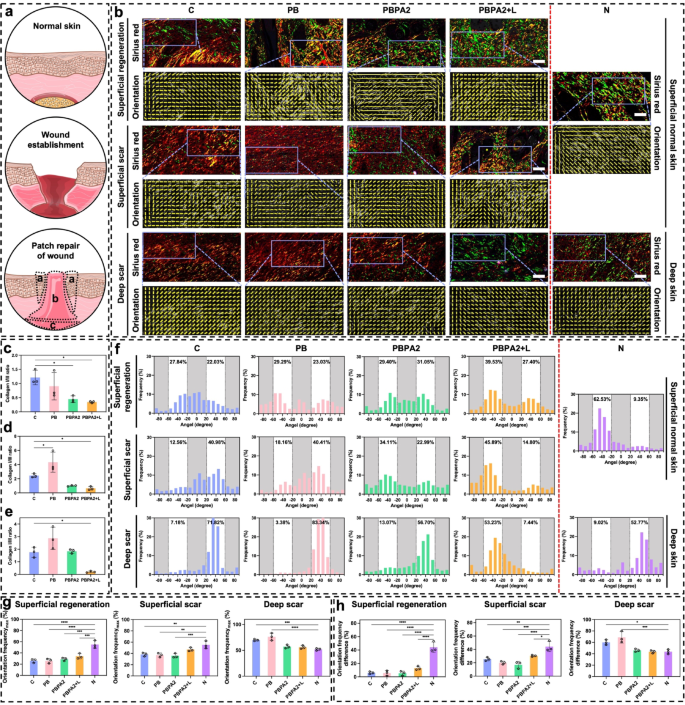
Picro-Sirius Red staining and healing orientation differences among three distinct healing parts. a) Schematic illustration of wound healing and the three different local healing area (a: Superficial regeneration-indicating full regeneration above dermal area; b: Superficial scar-indicating scar formation above dermal area; c: Deep scar-indicating scar formation below dermal area); b) Representative images of Picro-Sirius Red staining and orientation images on D14 in different treatment groups compare to normal skin based on the three different healing area, scale bar = 50 μm; c) Collagen I/III ratio of superficial regeneration area, n = 3; d) Collagen I/III ratio of superficial scar area, n = 3; e) Collagen I/III ratio of deep scar area, n = 3; f) Orientation distribution of different treatment groups compare to normal skin based on the three different healing area; g) Maximal single orientation frequency among different groups in three distinct regions, n = 3; h) Frequency difference between two different orientations among different groups in three distinct regions, n = 3. Data represented mean ± SD. **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05
In the superficial regeneration area of each group, although regeneration appeared similar in gross structure, the C group exhibited a more disorganized arrangement, and only the PBPA2 + L group achieved regeneration predominantly with type III collagen (Fig. 8b). Furthermore, inflammation or other undetected factors influenced the phase of collagen secretion. In group C, collagen I was predominant, whereas collagen III was more prominent under PBPA2 + L therapy. Although orientation distribution did not show significant improvement, it was highest in this region under PBPA2 + L treatment at 39.53% (Fig. 8f), indicating a more polarized healing pattern. Given that this region is located in the superficial structure, its polarity originated from the periphery rather than the underlying fascia. This suggests that the PBPA2 + L treatment promotes the peripheral normal tissue to regenerate towards the center, rather than the dense granulation tissue mobilized from the fascia beneath, thereby exhibiting higher distribution polarity.
Staining indicated that, except for the PBPA2 + L group, the repair mode in other groups was dominated by type I collagen-based patch repair, characterized by dense collagen structure in the superficial scar area. Only the PBPA2 + L group had a loose structure similar to the superficial skin of the N group (Fig. 8b). Moreover, the orientation distribution polarity at this site reached a maximum of 45.89% following PBPA2 + L treatment (Fig. 8f). These results suggest that in the central region of the wound, the slow phase transition and exacerbation of inflammation restrict the peripheral-to-central creeping boundary in groups other than the PBPA2 + L treatment. This restriction forces the dense mobilized fascia below to fill the gap upward, leading to the complete formation of scars incapable of regenerating normal structures. This bidirectional healing reduces distribution polarity. However, after PBPA2 + L treatment, this limitation is overcome, allowing a horizontal creeping pattern of healing from the periphery towards the center to dominate. This results in a distribution polarity at this level and a porous structure resembling normal skin, rather than the dense structure typical of patch repair. Although the distribution of collagen type III in this area is not as optimal as in the superficial regeneration area under PBPA2 + L treatment, it remains the best among the groups. This demonstrates the success of the treatment in replacing scar formation with skin regeneration.
The differences were more evident in the deep scar area. Orientation analysis suggested that, while the collagen orientation in all groups primarily exhibited a high single orientation distribution, the C and PB groups displayed an extremely high single orientation of 70%−80%, indicating the migration and repair of the wound crater by patch repair. This symmetry with the superficial area results in the deeper mobilized fascia in the C and PB groups attempting to fill upwards, leading to a much higher single orientation distribution. In contrast, under PBPA2 + L treatment, the healing pattern is dominated by an outside-in approach. This inhibits the migration of mobilized fascia in the deeper layers, resulting in a significant decrease in orientation distribution polarity. The PBPA2 + L group’s distribution decreased to a level comparable to normal skin at around 50% (Fig. 8b and f).
Finally, a quantitative analysis of the orientation distribution was conducted to evaluate differences in collagen alignment. The tissue was divided into three regions for assessment: superficial regeneration, superficial scar, and deep scar. First, the maximum values of the orientation distribution (Orientation distributionMax) from Fig. 8f were quantified (Fig. 8g). Among all three regions, the PBPA2 + L group exhibited a distribution closest to that of normal skin, with the most pronounced improvement observed in the deep scar area, where PBPA2 application alone already yielded values approaching those of normal tissue. Only the C and PB groups showed significantly higher values, consistent with the established role of deep fascia mobilization in scar formation. While the Orientation distributionMax reflects polarized alignment characteristics, it does not fully capture the overall structural organization. To better represent structural changes, the difference between the high and low polarization values was calculated (Fig. 8h). Although all groups still differed notably from Group N in the superficial regeneration area, the PBPA2 + L group achieved the highest value among the treated groups. Results in other regions aligned with those in Fig. 8g, showing mild improvement in superficial scar and, most notably, suppression of scar-prone deep fascia mobilization to a level comparable to normal skin. These findings demonstrate the strong scar-modulating capacity of the treatment.
Overall, in the superficial regeneration area, there was a predominantly peripheral-to-central crawling healing pattern, with all groups exhibiting skin regeneration. Consequently, each orientational distribution group showed a similar pattern at this site. However, the PBPA2 + L group had the highest single polarity, demonstrating the most singular directionality of healing in this area. This suggests that the inward migration was the most pronounced, serving as important evidence for the lack of scar growth in the final center. In the superficial scar area, there was a significant difference between the staining results among the groups. Only the PBPA2 + L group showed a good structure, correlating with the high polarity of its peripheral superficial regeneration area. Images from all other groups showed no normal dermal structure and a decrease in distribution polarity, likely due to the neutralization of the dual healing pattern of bottom-to-up and peripheral-to-central in this area. This change was evident in the deep scar area, where distribution polarity was high in the first two groups and fell back to around 50% after PBPA2 treatment. This suggests that migration in the deep area was greatly reduced, precisely because the peripheral-to-central migration in the superficial area was sufficient for wound healing without the need for deep tissue involvement. Taken together, the conclusion that PBPA2 + L treatment resulted in a change in spatial healing orientation was confirmed by Patch Repair Division Method analysis.
In vivo anti-inflammation effects of the treatments
The PBPA2 + L treatment promoted a peripheral-to-central migration pattern in the superficial area, closely linked to accelerated re-epithelialization. To evaluate keratinocyte proliferation at the wound edge, immunohistochemical staining for Ki67, a key proliferation marker, was conducted. Results demonstrated significantly elevated Ki67 expression in the basal layer of the epidermis in both PBPA2 and PBPA2 + L treatment groups at D7 (Fig. 9a and b). By D14, the PBPA2 group exhibited the highest Ki67 expression in the deep wound region (Fig. 9a and b), consistent with previous findings of excessive bottom-to-up patch repair in both superficial and deep scar areas. The proliferative surge correlated with localized neo-epidermal thickening, indicating Ki67-high disruptive hyperplasia rather than organized stratification. In contrast, the PBPA2 + L group showed moderated Ki67 and a thinner, more uniform epidermis, consistent with enhanced keratinocyte migration, accelerated wound closure, and timely remodeling. Earlier in vitro data indicated the treatment alters fibroblast-to-myofibroblast and M1-to-M2 macrophage differentiation, reducing reliance on myofibroblast-driven “bottom-up” dermal filling and promoting a looser, slower-growing basal structure. To further elucidate these mechanisms, following experiments were performed.
At D7, substantial inflammatory cell infiltration, including macrophages, persisted in the central wound area. To evaluate this, iNOS expression was examined in the mid-to-deep wound regions. Notably, the C and PB groups, lacking PDA-mediated anti-inflammatory/antioxidant therapy and AgNP-based antibacterial effects, showed significantly higher iNOS expression compared to the latter two treatment groups, a trend that persisted until D14 (Fig. 9c and d). Given that iNOS reflects both inflammation and M1 macrophage polarization, dual immunofluorescence staining for CD86 and CD206 was performed to validate the in vitro findings of PBPA-induced M2 polarization (Fig. 9e). By D14, the excessive M1 polarization shifted toward M2 polarization across all groups, attributable to ROS scavenging by PB, the combined anti-inflammatory and antibacterial effects of PBPA2, and the disruption of biofilm barriers by PBPA2 + L therapy. This shift was evident in both the M1/M2 ratio (Fig. 9f) and expression levels of M1 and M2 markers (Fig. 9g). Concurrently, tumor necrosis factor-α (TNF-α) levels at D14 further confirmed reduced inflammation following PBPA2 + L treatment (Fig. 9h and i). As previously observed, PBPA2 + L therapy effectively suppressed M1 polarization while promoting M2 polarization through dual antimicrobial and anti-inflammatory regulation, thereby converting the local pro-inflammatory microenvironment into an anti-inflammatory one. This anti-inflammatory milieu likely contributed to reduced activation of myofibroblasts, aligning with the observed decrease in TGF-β expression in the PBPA2 + L group at D14 (Fig. 9j and k). Furthermore, previous extensive evidence indicates that effective suppression of inflammation, exemplified here by the downregulation of iNOS and TNF-α, is essential for advancing the wound from the inflammatory to the proliferative phase. In parallel, polarization toward the M2 phenotype not only reinforces this transition through anti-inflammatory cytokines such as interleukin-10, but also provides pro-regenerative signals-VEGF and EGF-that stimulate fibroblast proliferation, collagen deposition, and neovascularization [69]. When coupled with the altered healing orientation induced by PBPA2 + L, these cues facilitate an orderly shift from proliferation into remodeling, ensuring that matrix deposition and tissue architecture evolve toward regeneration rather than fibrotic repair.
Collectively, these findings demonstrate that the PBPA2 nanozyme hydrogel therapeutic system mitigates the risk of post-infection inflammatory exacerbation in the wound microenvironment, thereby facilitating a phenotypic shift from M1 to M2 macrophage polarization. These findings were consistent with in vitro results. This reprogrammed macrophage population subsequently modulates downstream myofibroblast activation pathways, exhibiting the potential to suppress excessive TGF-β expression. The observed attenuation of local fibrosis and reduced Ki67 expression in the epidermal layer may collectively contribute to the transition from patch repair to a peripheral-to-central healing pattern. Ideally, the peripheral-to-central healing pattern could generate a tissue architecture more closely resembling native dermal-epidermal organization, potentially yielding substantially improved skin regeneration outcomes compared to deep patch repair. To evaluate the functional consequences of this healing orientation shift, subsequent validation was performed focusing on skin appendage regeneration and fibrosis parameters.
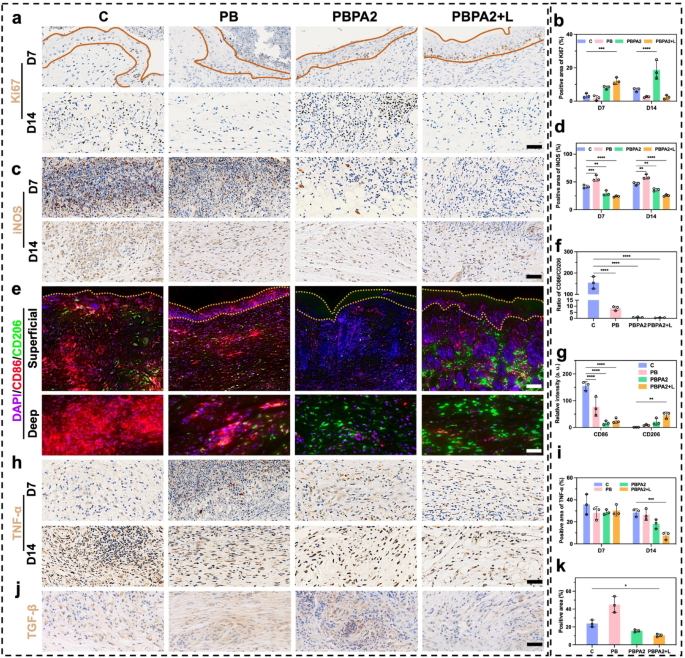
Immunofluorescence and immunohistochemistry staining of skin section under different treatment. a) Immunohistochemistry staining of Ki67 (area within yellow lines indicates epidermis), scale bar = 100 μm; b) Positive area of Ki67 based on immunohistochemistry staining, n = 3; c) Immunohistochemistry staining of iNOS, scale bar = 100 μm; d) Positive area of iNOS based on immunohistochemistry staining, n = 3; e) Immunofluorescence staining of CD86 and CD206 (area within yellow lines indicates epidermis), upper scale bar = 200 μm, lower scale bar = 100 μm; f) Ratio of CD86/CD206 based on immunofluorescence staining, n = 3; g) Relative intensity of CD86 and CD206 based on immunofluorescence staining, n = 3; h) Immunohistochemistry staining of TNF-α, scale bar = 100 μm; i) Positive area of TNF-α based on immunohistochemistry staining, n = 3; j) Immunohistochemistry staining of TGF-β on D14, scale bar = 100 μm; k) Positive area of TGF-β based on immunohistochemistry staining, n = 3. Data represented mean ± SD. **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05
Skin regeneration improved after healing orientation changes
Scar formation is closely linked to myofibroblast activation, marked by α-SMA and vimentin co-expression [70]. This process primarily arises from patch repair, involving upward migration of deep tissue [68]. Since the hair-bearing superficial dermis complicates fibrosis evaluation, we focused subsequent analysis on deep patch repair tissue. Immunofluorescence co-staining revealed maximal myofibroblast abundance at D14 in the PB and PBPA2 groups, which correlated with severe scarring. In contrast, the C group showed weaker vimentin expression, consistent with its impaired healing. In the PBPA2 + L group, co-expression of α-SMA and vimentin was significantly lower (Fig. 10a). Semi-quantitative colocalization analysis revealed low vimentin expression in the C group (Fig. 10b), high fluorescence intensity of vimentin and α-SMA in the PB and PBPA2 groups (Fig. 10c and d), and low fluorescence intensity and overlapping peaks in the PBPA2 + L group (Fig. 10e). Similarly, Pearson’s ratio and Overlap ratio indicated that the PBPA2 + L treatment group had the lowest levels of fibrotic myofibroblast activation, while the groups with pronounced scarring had the highest (Fig. 10f and S41). Reduction in vimentin may be due to a decrease in the bottom-to-up healing pattern, but, of course, there are multiple other factors besides this. First, transient myofibroblasts in the treatment do not transition to persistent myofibroblasts, thus rapidly repairing the wound in the early phase and reducing activation during the remodeling phase, promoting skin regeneration instead of scar formation [49]. Second, it is also possible that clearance of ROS can lead to vimentin expression downregulation and decreased downstream TGF-β expression which was confirmed in Fig. 9j [71]. This observation may also correlate with our earlier in vitro findings that PDA@AgNPs exert antibacterial effects while modulating macrophage polarization, consequently downregulating TGF-β expression in downstream fibroblasts (Fig. 5f). Additionally, reduced vimentin resulted from decreased type I collagen secretion [71]. Subsequently, the reduced myofibroblast activation resulted in a more loosely organized tissue structure conducive to remodeling. Within this pro-regenerative microenvironment, significantly upregulated expression of the vascular marker CD31 was observed (Fig. 10g-h and S42). Such vascular remodeling is suggestive of functional neovascularization, which is critical for restoring microcirculatory support, ensuring adequate perfusion and nutrient exchange, and thereby sustaining the metabolic demands of regenerating skin tissue.
Since the superficial dermis is the primary region for hair follicle regeneration, analysis on this area to evaluate skin regeneration outcomes was primarily focused. Following enhanced angiogenesis and structural remodeling, a higher number of skin appendages in this layer in the PBPA2 + L group was determined (Fig. 10i and j). Macroscopic analysis of the regenerated hair area also reflected similar results (Fig. 10k). α-SMA and β-catenin are markers highly associated with hair follicle neogenesis. Co-immunofluorescence staining for α-SMA and β-catenin allows clear observation of new hair follicle development [70]. The PBPA2 + L group exhibited an upregulated β-catenin signal in the superficial dermis compared to the C group, and the results were highly similar to the normal group (N) (Fig. 10l). Semi-quantitative colocalization analysis indicated more overlapping peaks of β-catenin and α-SMA signal in the PBPA2 + L group compared to the C group, and similar to the N group. Additionally, the fluorescence intensity of β-catenin in both groups was higher than in the C group (Fig. 10m and o). Quantitative colocalization indices, including Pearson’s ratio and Overlap ratio, showed that hair follicle neogenesis in the PBPA2 + L group surpassed that of the C group, reaching a level comparable to normal skin (Fig. 10p and S43). The underlying mechanisms are likely multifactorial. During the remodeling phase of wound healing, the optimization of tissue architecture and enhanced angiogenesis provide critical microenvironmental support for hair follicle regeneration [72, 73]. As collagen fibers realign and the ECM undergoes dynamic remodeling, the initially disorganized scar tissue is progressively replaced by more physiologically functional connective tissue. This structural improvement not only reinforces mechanical support for perifollicular tissues but also establishes a more favorable growth niche for hair follicle stem cells [74, 75]. Importantly, the β-catenin/α-SMA co-localization observed here might aligns with activation of the follicular stem cell niche, a prerequisite for initiating new anagen cycles [76]. By reawakening quiescent stem cells within a supportive microenvironment, the regenerative process extends beyond follicle formation to the restoration of functional hair-bearing skin. Concurrently, a robust network of nascent capillaries forms densely under sustained stimulation by growth factors such as VEGF, significantly improving local blood perfusion and nutrient supply. These newly formed vessels not only deliver oxygen and essential nutrients but also transport various cytokines and signaling molecules that promote hair follicle activation [77]. Within this highly vascularized microenvironment that more closely resembles native tissue, quiescent hair follicle stem cells are reactivated, exhibiting markedly enhanced proliferative and differentiation capacities that drive follicles into new anagen cycles. Furthermore, specific ECM components, including laminin and fibronectin secreted by activated fibroblasts during remodeling, form a three-dimensional scaffold that supports dermal papilla cell aggregation and follicular morphogenesis, ultimately facilitating the regeneration of more robust hair structures [78]. The synergistic interplay between tissue remodeling and angiogenesis essentially reconstructs a follicle microenvironment resembling native tissue, establishing both the structural foundation and physiological conditions necessary for functional hair regeneration.
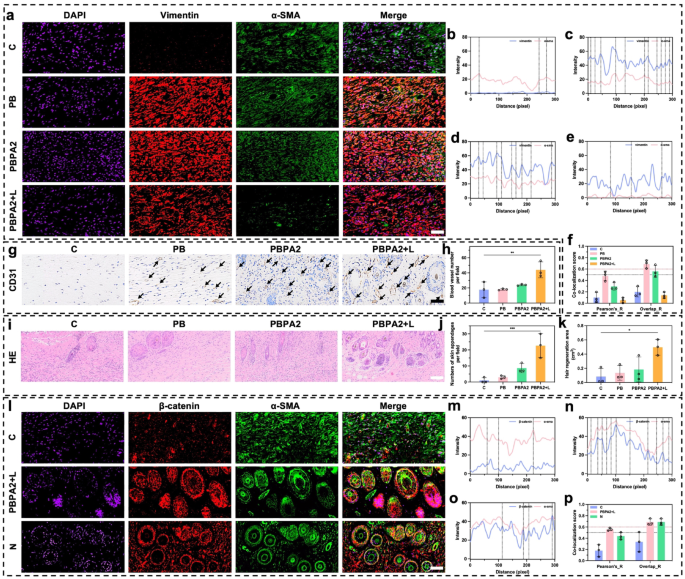
Evaluation of skin appendages regeneration and fibrotic scar formation after healing orientation change. a) Immunofluorescence staining of DAPI, vimentin, and α-SMA in deeper dermis on D14, scale bar = 50 μm; b) Immunofluorescence colocalization analysis of vimentin and α-SMA in control group; c) Immunofluorescence colocalization analysis of vimentin and α-SMA in PB group; d) Immunofluorescence colocalization analysis of vimentin and α-SMA in PBPA2 group; e) Immunofluorescence colocalization analysis of vimentin and α-SMA in PBPA2 group; f) Colocalization index calculated through vimentin and α-SMA immunofluorescence staining in different groups, black line indicates threshold of Pearson’s ratio which is 0.5, red line indicates threshold of Overlaps ratio which is 0.6, n = 3; g) Immunochemistry staining of CD31 on D14, black arrows indicate blood vessels, scale bar = 100 μm; j) Blood vessel number per field calculated on immunochemistry staining, n = 3; i) Skin appendages regeneration in different treatment groups on H&E staining, scale bar = 100 μm; j) Number of skin appendages on D14 per field calculated on H&E staining, n = 3; k) Area of hair regeneration on D14 of mice, n = 3; l) Immunofluorescence staining of DAPI, β-catenin, and α-SMA in superficial dermis on D14, scale bar = 50 μm; m) Immunofluorescence colocalization analysis of β-catenin and α-SMA in control group; n) Immunofluorescence colocalization analysis of β-catenin and α-SMA in PBPA2 + L group; o) Immunofluorescence colocalization analysis of β-catenin and α-SMA in normal skin; p) Colocalization index calculated through β-catenin and α-SMA immunofluorescence staining in different groups, black line indicates threshold of Pearson’s ratio which is 0.5, red line indicates threshold of Overlaps ratio which is 0.6, n = 3. Data represented mean ± SD. **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05
These results hint that a peripheral-to-central dominant healing pattern after the treatment may provide a better foundation for skin regeneration. This includes increased vascularity, more localized appendage regeneration, and upregulation of follicle neogenesis-related expression. These improvements are mainly due to the inhibition of bottom-up patch repair and significantly reduced fibrosis, likely resulting from the dual action of inflammatory control and antibacterial, which normalizes collagen distribution and ECM arrangement downstream. Finally, with restraining fibroblast-to-myofibroblast transition and mitigating sustained profibrotic signaling, this modulation not only facilitates orderly ECM remodeling but also preserves a dermal architecture that is less densely fibrotic and more physiologically aligned. Such structural normalization is expected to restore appropriate collagen fiber orientation and improve tensile strength, thereby reinforcing the functional integrity of regenerated skin.
In vivo biocompatibility of PBPA hydrogel
After achieving promising therapeutic effects, in vivo biotoxicity and compatibility were evaluated. At the same time, excessive deposition of silver is thought to bring about in vivo toxicity, and in order to rule out such a risk, the following experiments were carried out.
First the actual amount of Ag applied were calculated as around 2.5 mg for each wound. This is a localized application, and the actual amount entering the systemic circulation may be even lower. Studies have shown that mice consuming 110 mg of silver over one week exhibit no adverse effects [79, 80]. For humans, the World Health Organization states that a lifetime intake of 10 g of silver poses no health risks [80]. Clinical study also indicates that daily ingestion of 50 mg of silver does not cause any adverse effect of silver toxicity [81]. Furthermore, certain populations naturally have silver levels as high as 100 g in their bodies [80]. Therefore, using 2.5 mg of silver to treat a wound of approximately 0.5 cm² is unlikely to have significant adverse effects. To better picture, tracking body weight data throughout the treatment period showed a steady increase in all groups, with the PBPA2 + L group exhibiting the highest weight gain and the C group the least (Fig. S44). Hematological (Fig. S45) and biochemical (Fig. S46) analyses conducted on D14 indicated that the hydrogel components in each group did not cause any significant toxic changes in the measured parameters. Notably, some indicators in the C group remained outside the normal range. This systemic biocompatibility was further confirmed by H&E staining of major organs, including the heart, liver, spleen, lungs, and kidneys (Fig. S47).
These results demonstrate that PBPA2 hydrogel-guided antibacterial nanozyme treatment exhibits excellent biocompatibility in vivo, with no detectable systemic toxicity. Overall, this therapeutic program offers precise, phase-specific control over wound healing while addressing the structural limitations of conventional treatments. In the hemostatic phase, PBPA2 + L rapidly achieves bleeding control, debridement, thermal therapy, and biofilm disruption. During inflammation, ROS sensing triggers the controlled release of nanozymes, enabling efficient ROS and bacterial clearance, which shortens the inflammatory phase and promotes transition to the proliferative phase. In the proliferative phase, suppression of myofibroblast overactivation, combined with the hydrogel’s low modulus and degradability, creates a favorable niche that accelerates re-epithelialization, fibroblast proliferation, and collagen deposition. Finally, in remodeling, these properties interrupt the stress–myofibroblast loop, enabling efficient ECM reorganization and appendage regeneration. Unlike standard wound therapies that target only one aspect, antibiotics for bacterial clearance, antioxidants for ROS scavenging, or dressings that provide passive coverage, the hydrogel integrates these therapeutic dimensions into a single spatiotemporally programmed system. By dynamically balancing ROS levels, it resolves the dilemma of exploiting ROS for antibacterial action while preventing oxidative tissue damage. Moreover, by shifting healing from a bottom-up, scar-filling trajectory to a peripheral-to-central regenerative mode, it establishes an advanced paradigm that enables true skin regeneration rather than incomplete closure.
Nevertheless, this study has several limitations that warrant further investigation: (1) While altered healing orientation was associated with elevated Ki67 expression and modified fibroblast activity, the precise mechanisms-potentially involving collagen ratio shifts and vimentin-mediated remodeling-remain to be clarified. (2) The programmed treatment design, though effective, still requires optimization, and manual NIR irradiation compromises stability compared to autonomous platforms, underscoring the need for integrated and clinically safe delivery systems. (3) Scalability and clinical translation remain challenging, as large-area or deep wounds may demand uniform hydrogel application and precise ROS modulation in heterogeneous environments. (4) Moreover, although biofilm-infected wounds were emphasized, diabetic wounds represent another critical but unaddressed chronic model, highlighting the need for broader validation before clinical translation.

